The appropriateness of use of BPA in some consumer products has been questioned after recurrent reporting of unfavourable health effects of BPA. Government organisations of many countries have probed into the matter and imposed regulations on marketing of plastic stuff containing BPA [15]. Restrictions imposed on the use of BPA have led to a shift towards the use of its analogues like Bisphenol S (BPS) which has been introduced as a harmless substitute to BPA by various plastic manufacturers. It is increasingly replacing BPA in a variety of consumer goods [16]. BPS is being investigated for its effects on various body systems and has been found to cause ill effects on reproductive, endocrine and nervous system and to generate oxidative stress [17]. Opposing to BPA, it is still not established if BPS affects gut motility. Therefore, the present study was undertaken to evaluate and compare the effects of chronic ingestion of BPA and BPS on gut motility. Accordingly the objective was to examine, in-vitro, spontaneous and Acetylcholine (Ach)-induced gastric and small intestinal contractions in BPA and BPS fed rats.
Materials and Methods
Animals and Groups
This animal experimental study commenced after ethical clearance from the institutional ethical clearance committee (Ref. No. Dean/2017/CAEC/245) and was conducted in 2018 (October-December) taking all ethical considerations for animal studies. Duration of study was about three months. Adult male albino rats of Charles foster strain (weighing 175-225 g) were procured from institutional animal house. The rats were fed standard laboratory feed and water ad libitum, and were maintained in the departmental animal house in conditions of controlled temperature (25±1°C) and light (12:12 hr light dark).
The study was conducted on total 18 rats. The rats were divided in to three groups (n=6 in each group). From each rat in a group, one gastric and one small intestinal muscle strip was prepared. So in a group from total 6 rats, 6 gastric and 6 small intestinal strips were prepared.
Group I was fed BPA (50 mg/kg/day in 0.5 mL of 20% Dimethyl sulphoxide, DMSO), Group II was fed BPS (50 mg/kg/day in 0.5 mL of DMSO), and group III was fed vehicle (0.5 mL/day of 20% DMSO), for four weeks by oral gavage besides the normal food and water. Group III served as control.
Dissection of Animal
On 29th day, after overnight fasting, rats were sacrificed by cervical dislocation. Quickly after sacrificing the animal, the abdomen was opened by mid line incision. The stomach and small intestine were dissected out and placed in a petridish containing 100% oxygenated fresh Krebs-Ringer solution. The small intestinal contents were flushed out by this solution with the help of a syringe. Small intestinal segment (10-15 mm long) was opened along the length and thin muscle strips were prepared. The stomach was opened along the greater curvature and the contents were flushed out. Thick and muscular corpus part of stomach was used to prepare 10-15 mm long gastric muscle strips.
Mounting and Recording of Contractile Response
Mounting and recording techniques for smooth muscle strips were performed as described earlier from our laboratory [18]. Caliberation for the tension (0-10 g) was performed at the beginning and end of each recording. The muscle strips (10-15 mm) were placed in an organ bath (50 mL) filled with Krebs-Ringer solution (37°C±0.5°C). The solution was continuously bubbled with 100% O2. The muscle strip was secured to a tissue holder at one end, and to a force transducer (MLT 0210, AD instruments, Australia) on the other. Strips were mounted vertically for primarily recording of contractions of longitudinal muscle. The tissue segment was placed under optimum resting tension, 0.5 g for small intestinal muscle strips and 1.0 g for gastric muscle strips, and then left to equilibrate for 30-45 minutes, with replacement of Krebs-Ringer solution every 15 minutes to avoid accumulation of metabolites.
Isometric contractions of gut muscle strips were recorded in an organ bath preparation (37±0.5°C) using force transducer, computerised polygraph power lab (4ST) and chart-5 for Window, ADI, Sydney, Australia. After recording, the muscle strip was removed from the organ bath and placed on blotting paper for lightly soaking the extra water from the tissue. The two ends of the strips were discarded to avoid the damaged tissue. The wet tissue was then weighed.
Drugs and Solutions
The physiological solution (Krebs-Ringer solution) was prepared with following compositions (in mmol): NaCl, 119; KCl, 4.7; CaCl2.2H2O, 2.5; KH2PO4, 1.2; MgSO4.7H2O, 1.2; NaHCO3, 5; and glucose, 11, with pH adjusted to 7.4. ACh, BPA and BPS were obtained from Sigma Aldrich, US. Bisphenols were dissolved in 20% Dimethyl Sulphoxide (DMSO). DMSO was obtained from Merck, Mumbai. The stock solution of ACh (100 mM) was prepared in distilled water and the final dilutions were made in Krebs-Ringer solution.
Experimental Protocol
The tissue was allowed to stabilise for a minimum duration of 30 minutes for small intestinal segment and 45 minutes for gastric segments. Later, the spontaneous contractile activity was recorded for 10 minutes. Thereafter, tissue was exposed to cumulative concentration of ACh (0.1-100 μM) to obtain the dose response. The tissue was exposed to each dose of ACh for 10 minutes. At the end of each experiment, the tissue segment was removed, blotted and weighed.
Parameters Studied
The contractions of gut muscle strips were evaluated in terms of three parameters: 1) Maximum tension attained per unit weight during each contraction (MCT, Maximum Contractile Tension); 2) Number of contractions per minute (CF, Contractile Frequency); and 3) Basal level of tension reached per unit weight after each contraction (BT, Basal Tone).
The maximum height of contractions were converted to tension (g) with help of chart-5 software and was expressed as MCT per unit mass (g/g wet tissue) using the tissue weight determined at the end of the experiments. CF was calculated as contractions per minute. Similarly, basal tension (at end of each contraction) was expressed as BT per unit mass (g/g wet tissue) of tissue. Spontaneous contractile activity was recorded for 10 minute and data of one minute was evaluated (9th to 10th minute). The spontaneous contractile activity was considered as 100% and dose response of Ach was expressed in terms of percentage of initial value of spontaneous contractile activity. The Ach response was in form of increase in contractile tension as well as BT. Therefore, the point of time with maximum increase in the parameters after each cumulative dose of Ach was selected for assessment of Ach dose response.
Statistical Analysis
The values were pooled to calculate Mean±SEM. One-way ANOVA and two-way ANOVA were applied as required for multiple comparisons and p-value <0.05 was considered statistically significant.
Results
1. Assessment of In-vitro Contractile Activity of Gastric Muscle Strips
a. Spontaneous contractile activity of gastric muscle strips in control group
The spontaneous contractile activity started within 45 minutes during stabilisation period and remained for a prolonged period. The contractions observed were slow and tonic type. The contractions varied from strip to strip in their amplitude and frequency. The absolute Mean±SEM values of BT, MCT and CF were observed as shown in [Table/Fig-1]. [Table/Fig-2] shows the original sample recording of spontaneous contractile activity of gastric tissue in control group.
Mean±SEM of values of contractile frequency, maximum contractile tension (per g wt.) and basal tone (per g wt.) of gastric muscle strips in control, BPS fed and BPA fed groups obtained from recordings of spontaneous contractions. Asterisk indicates significant difference from control group (p<0.05, one-way ANOVA).
| Groups (n=6) | Contractile frequency | Maximum contractile tension/g wt | Basal tone/g wt |
|---|
| Control | 2.19±0.12 | 11.28±1.44 | 9.58±0.96 |
| BPS fed | 2±0.16 | 8.89±1.35 | 7.87±0.98 |
| BPA fed | 1.51±0.46 | 4.75±0.40* | 4.37±0.44*‡ |
‡indicate significant difference between BPA and BPS fed group (p<0.05, one-way ANOVA)
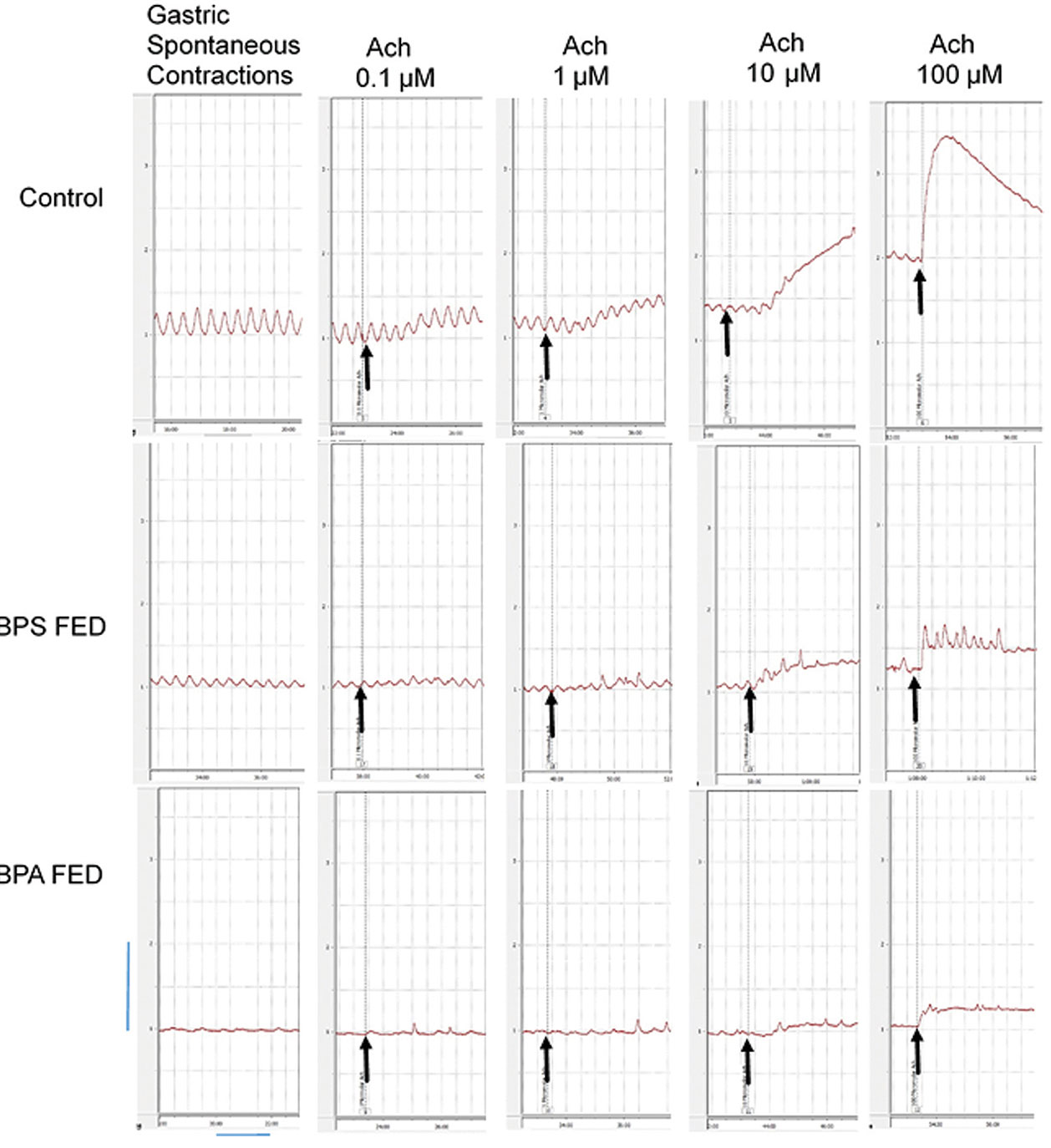
Sample of original recordings from gastric muscle strips before and after application of different bath concentrations (0.1, 1, 10 and 100 μM) of Ach in control, BPS fed and BPA fed groups.
Arrows indicate the point of application of drug, vertical and horizontal calibrations represent the tension (g) and time (min) respectively. Vertical bar is equivalent to 1 g tension and horizontal bar is equivalent to 2 minutes.
b. BPA treatment significantly diminished spontaneous contractile activity of gastric muscle strips as compared to control
The absolute initial values of CF, MCT and BT of gastric muscle strips in different groups [Table/Fig-1] were compared (one-way ANOVA). The initial contractile frequency in all three groups was similar (p>0.05). The MCT and BT of BPA fed group was significantly less than (p<0.05) control group. The mean value of initial MCT and BT of BPS fed group, was less than control but the difference was not statistically significant. When both the treated groups (BPA fed and BPS fed) were mutually compared, the MCT was similar but the BT was significantly less in BPA fed group as compared to BPS fed group.
c. Ach evoked contractions in gastric muscle strips were attenuated in both the treated groups as compared to control.
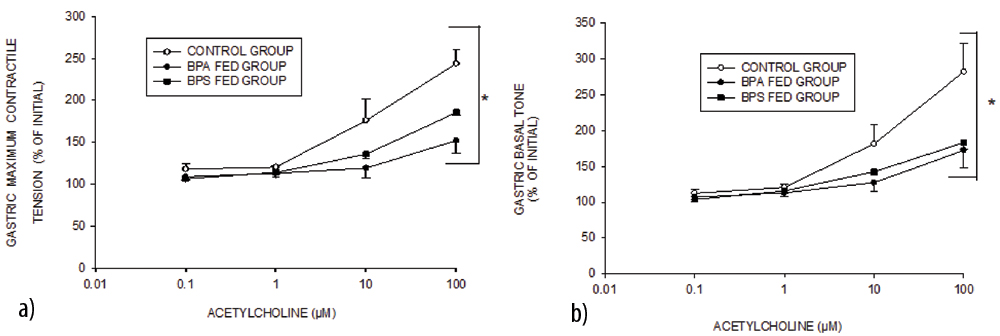
When treated with 0.1,1 and 10 μ M Ach, the response of gastric muscle strips was similar in all the three groups. With 100 μM Ach, the response of BPA fed group was significantly less as compared to control. The BPS fed group responded to 100 μM Ach in a manner similar to BPA fed group [Table/Fig-2,3]. The Ach dose response of both BPA fed and BPS fed group was significantly different from the control group (p<0.05, two-way ANOVA). Gastric muscle strips responded to Ach treatment in form of rise in MCT [Table/Fig-3a] as well as BT [Table/Fig-3b].
Mean±SEM values of maximum contractile tension (/g wt.) and basal tone (/g wt.) in control, BPS fed and BPA fed groups (n=6 in each group) achieved in response to different cumulative (0.1 μM-100 μM) doses of acetylcholine in gastric muscle strips.
Data expressed as % of initial tension. The asterisk indicates significant (p-value <0.05, two-way ANOVA) difference from control
2. Assessment of In-vitro Contractile Activity of Intestinal Muscle Strips
a. Spontaneous contractile activity of intestinal muscle strips in control (vehicle treated) group
The spontaneous contractile activity started within 30 min during stabilisation period and persisted for a long period. The frequency of intestinal contractions was more than that of gastric and the contractions were of phasic type. Like the gastric contractions, the intestinal contractions also varied from strip to strip in their amplitude and frequency. The absolute Mean±SEM values of BT, MCT and CF are presented in [Table/Fig-4]. The original sample recording of spontaneous contractile activity of small intestinal tissue in control group is shown in [Table/Fig-5].
Mean±SEM of values of contractile frequency, maximum contractile tension (per g wt.) and basal tone (per g wt.) of small intestinal muscle strips in control, BPS fed and BPA fed groups.
| Groups (n=6) | Contractile frequency | Maximum contractile tension/g wt | Basal tone/g wt |
|---|
| Control | 13.71±0.52 | 7.37±1.36 | 6.55±1.16 |
| BPS fed | 13.09±1.01 | 4.38±0.55* | 3.69±0.64* |
| BPA fed | 11.25±0.59 | 2.65±0.2* | 2.43±0.16* |
Asterisk indicates significant difference from control group (p<0.05; one-way ANOVA)
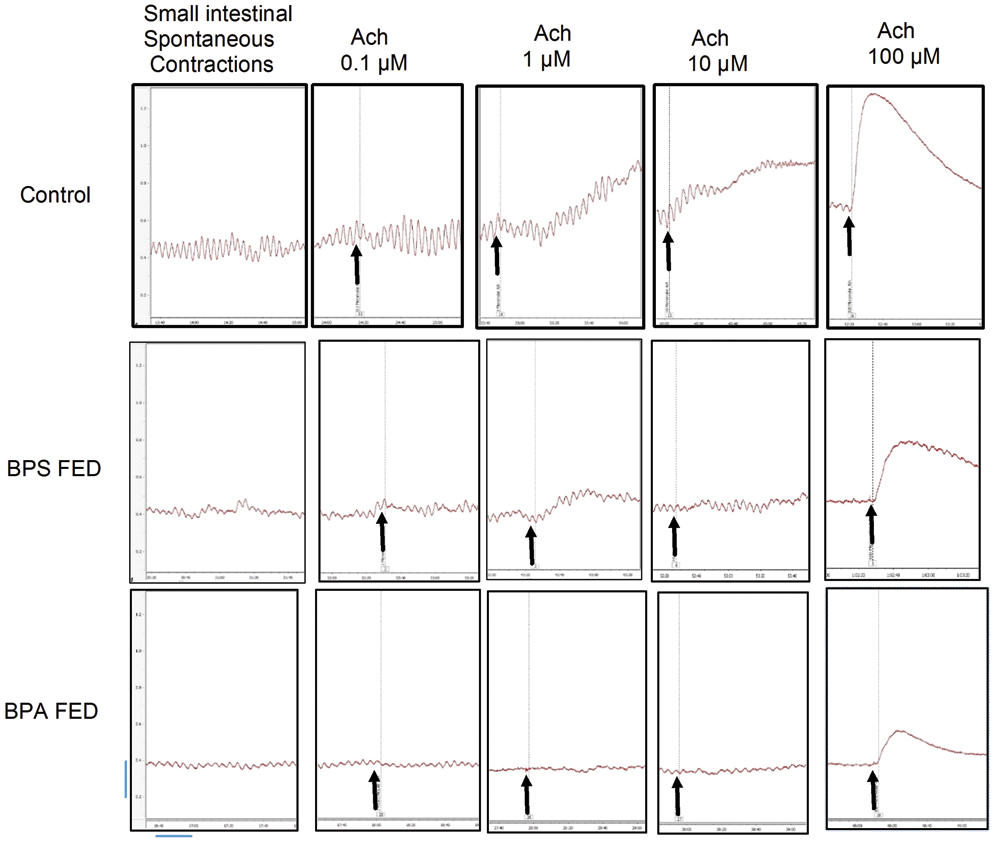
Samples of original recordings from small intestinal muscle strips before and after applying different concentrations (0.1, 1, 10 and 100 μM) of Ach in control, BPS fed and BPA fed groups.
Arrows indicate the point of application of drug, vertical and horizontal calibrations represent the tension (g) and time (min) respectively. Vertical bar is equivalent to 0.2 g tension and horizontal bar is equivalent to 20 seconds
b. BPA and BPS treatment significantly diminished spontaneous contractile activity of intestinal muscle strips
The absolute initial values of CF, MCT and BT of small intestinal muscle strips in different groups [Table/Fig-4] were compared (one-way ANOVA). The initial CF in all three groups was similar (p>0.05). The MCT and BT of both BPA and BPS fed group were significantly less than control group. When both the treated groups were mutually compared, MCT and BT both were similar to each other.
c. Ach evoked contractions in small intestinal muscle strips were attenuated in both the treated groups
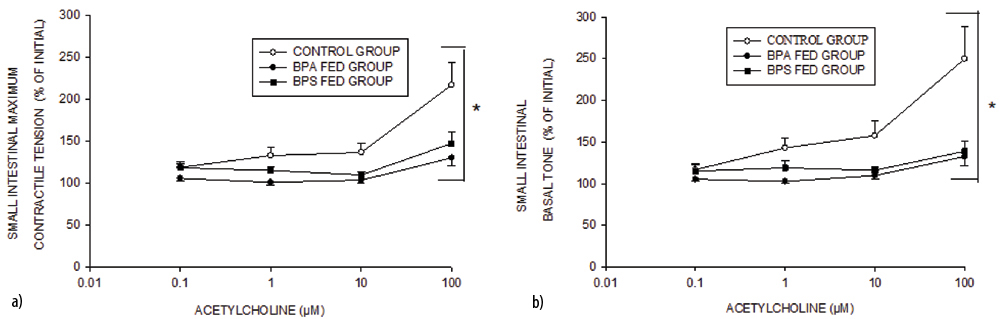
Ach response was in form of rise in MCT [Table/Fig-6a] as well as BT [Table/Fig-6b]. With lowest bath concentration of Ach (0.1 μM) all the groups responded in a similar manner. With 1μM Ach bath concentration, the Ach response of BPA fed group was significantly less than control. At higher bath concentrations, (10 and 100 μM), the Ach response of both the treated groups were significantly less as compared to control. The Ach dose response of BPS fed group was not different from that of BPA fed group [Table/Fig-5,6]. The Ach dose response of both BPA fed and BPS fed group was significantly different from the control group (p<0.05, two-way ANOVA).
a, b Mean±SEM values of maximum contractile tension (/g wt. and basal tone (/g wt.) in control, BPS fed and BPA fed groups (n=6 in each group) achieved in response to different cumulative (0.1 μM-100 μM) doses of acetylcholine in small intestinal muscle strips.
The asterisk indicates significant (p-value <0.05, two-way ANOVA) difference from control group
Discussion
The present study investigated the effect of chronic ingestion (50 mg/kg/day) of BPA and BPS on in-vitro gastric and small intestinal contractility to understand differential impact of BPA and BPS on contractile function of gut smooth muscle.
Our findings indicated that the chronic ingestion of BPA attenuated the spontaneous contractile activity and the Ach-induced contractions in small intestine as well as stomach. On the other hand, chronic ingestion of BPS inhibited spontaneous contractile activity only in intestine and not in stomach. However, the Ach-induced contractions were reduced in both intestine and stomach. There are a few studies [11-13] showing the impairment of in-vitro intestinal contractility in rats following chronic BPA ingestion. The present study for the first time demonstrated that chronic BPA ingestion may depress gastric contractility as well. Also, this is the pioneer study reporting the inhibition of contractile activity of gut smooth muscle following chronic ingestion of BPS, a substitute to BPA. The study also observed the comparative impairment of gastric and small intestinal gut contractility by BPA and BPS. The comparison of effects of BPA and BPS indicated that both BPA and BPS depressed spontaneous and Ach-induced contractions in small intestine and stomach except that BPS did not affect the spontaneous contraction in stomach significantly.
It was observed that the depression of spontaneous contractile activity by both the bisphenols was characterised by reduction of MCT as well as BT. The observations, therefore, signified that bisphenols might affect contractile machineries. Although, the frequency of spontaneous contractions, which is primarily a function of ICC (interstitial cells of Cajal) [19] was not affected significantly which suggests sparing of ICC by both the bisphenols.
Both the bisphenols are known EDC [3] and have oestrogenic actions [4]. Oestrogen has been found to impair gut contractile activity [20]. Therefore, the decreased contractile functions of gut may be ascribed to the oestrogenic actions of bisphenols. Oestrogen is known to act via two types of oestrogen receptors (ER), namely ERα and ERβ. The ERβ is known to be expressed in intestine [21]. However, pretreatment with an oestrogen receptor antagonist, tamoxifen could not block the BPA induced inhibitory responses in rat ileum and colon [22]. Therefore, it is still not clear if the inhibitory effect of BPA is mediated by a mechanism involving ER receptor, at least in rat gut.
Oestrogen has some ER independent actions as well through activation of potassium channels or inhibition of calcium channels [23]. Therefore, inhibitory response of bisphenols on gut contractility could also be brought about by its oestrogenic, but ER-independent action on gut smooth muscle.
Nitric Oxide (NO) was reported to be involved in BPA induced attenuation of in-vitro contractile activity of atria [24] and uterus [25]. However, in another study NO inhibitor L-NAME synthatase failed to block the inhibitory effect of BPA on gut contractility [22].
Besides this, BPA has also been reported to inhibit duodenal movement by increasing AChE activity, decreasing the availability of free Ca2+ in smooth muscle cells [11] and via α-adrenergic signaling pathways in visceral smooth muscle cells [14].
In present study, ACh evoked small intestinal contractions which were similarly attenuated in both BPA and BPS fed rats. This indicates modulation of cholinergic responses by both bisphenols. The action of acetylcholine is known to involve M3 and IP3/DAG signaling mechanism [26]. Diminished contractile response to Ach in BPA fed rats has also been reported in uterine tissue earlier [27].
The exact mechanism by which BPA inhibits gut contractility is yet to be established. The present investigation demonstrated that BPS depresses the gut contractility in a manner similar to that of BPA. Therefore, it is possible that it may affect the same contractile machineries of gut smooth muscle by similar pathways if not identical or some additional mechanism.
Due to excellent stability against high temperature, resistance to sunlight and significantly lower oestrogenic activity as reported by some earlier studies, BPS was once considered as a safe substitute of BPA [28]. However recent studies indicated that BPS has hormonal potencies similar to BPA [17]. Further, BPS been shown to share many in-vitro and in-vivo toxicities with BPA, rather in some studies, BPS has been found to show broader toxicological characterization and may bring additional changes which may cause disturbances not seen with BPA. BPS, contrary to BPA, increased 17α-OH progesterone levels [29] which is known to depress gut motility [30].
Limitation
The mechanism of action of BPA and BPS could be better evaluated by incorporating more number of agonists like seratonin and histamine (in addition to acetylcholine). Further, there is definite scope of assessment of long term effects of plastic toxins by increasing the duration of feeding and by exposing the rats to different doses of these toxins.
Conclusion
Both BPS and BPA have potential to decrease the gut contractility which may result into gut dysmotility. Therefore consideration of BPS as a safe substitute to BPA needs to be revisited with more careful approach.