Introduction
Nonalcoholic Fatty Liver Disease (NAFLD) is characterised by fat accumulation in the liver. Treatment of NAFLD in children is an important issue but the options are limited. Green tea has antioxidant and anti-hyperlipidemic effects but studies; on the effect of green tea in children are limited.
Aim
To investigate effect of green tea on NAFLD in children.
Materials and Methods
In this study, 52 children aged 10-16 years with NAFLD were divided into two groups of 26 each: Intervention and control. For both groups, modification of diet and intensification of physical activity were prescribed for three months. Intervention group was also treated with green tea tablets. Sonography and measurements of liver enzymes {Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST)}, lipid profile and Body Mass Index (BMI) were conducted before and after the intervention. The data were analysed by the Statistical Package for the Social Sciences (SPSS) version 19.0 using ANCOVA.
Results
After treatment with green tea, fatty liver grade decreased significantly in the intervention group compared to control group (p<0.0001). In addition, this treatment caused significant decrease in ALT, AST, and triglyceride levels and significant increase in High-Density Lipoprotein (HDL) level (p<0.05), but Low-Density Lipoprotein (LDL) level did not decrease significantly in the intervention group compared to control.
Conclusion
Oral prescription of green tea was effective in improving fatty liver grade, decreasing hepatic fat accumulation and improving liver function, weight loss and reducing ALT and AST without any side effects. These effects can be due to green tea compounds such as polyphenols especially catechin and antioxidant and anti-hyperlipidemic effects.
Introduction
NAFLD is characterised by hepatic fat accumulation despite low amounts of alcohol intake, presenting with hepatomegaly signs [1]. NAFLD is a metabolic disorder frequently diagnosed in western and eastern countries, resulting in increased liver and cardiovascular disease-related morbidity and mortality [2]. The prevalence of NAFLD is 34% in adults [3]. Most NAFLD patients are 40-60 years-old but children aged over 10 years are also likely to develop NAFLD [4].
NAFLD is now considered an independent condition as with obesity, hypertension and diabetes. It is widely acknowledged that NAFLD has close association with central obesity. However, 20-40% of NAFLD patients are not obese. It has been widely accepted that hyperlipidaemia leads to NAFLD [1].
The cause and treatment for NAFLD have not yet been definitely determined [1]. Early diagnosis of this disease is essential and its treatment can prevent progression to cirrhosis [5,6]. Lifestyle interventions such as exercise and weight loss are the only widely accepted treatments for this disease but they are difficult to adhere to for most NAFLD patients [5].
Camellia sinensis (Green tea) is a medicinal plant that originated from China and South East Asia thousands of years ago and is one of the most widely known beverages across the world [7].
In green tea drinkers, a significant reduction has been reported in the risk of developing hepatic diseases such as hepatocellular carcinoma, liver cirrhosis, liver steatosis, chronic liver disease and hepatitis [8]. C. sinensis is one of the richest sources of flavonoids with different health benefits [9].
Green Tea Polyphenol (GrTPs) have different health benefits [9]. GrTPs have antimutagenic, antioxidant, antidiabetic, antibacterial and anti-inflammatory properties [10]. GrTPs prevents hyperlipidaemia in the liver and causes increase in energy consumption, oxidation of fat and decrease in the body fat percentage [11]. In animals on high-fat diets that received C. sinensis and GrTPs, lower Triglyceride (TG) and cholesterol levels and higher faecal fat levels compared to controls were observed [12]. C. sinensis therefore has potential to control the weight of obese people. In vitro, C. sinensis extract is involved in the process of lipid emulsification, inhibition of fatty acids activity and synthesis as well as decrease in the hepatic fat accumulation [5,13].
The main polyphenolic compounds of C. sinensis are catechins, such as Epigallocatechingallate (EGCG), epigallocatechin, epicatechingallate, and epicatechin, which have been reported to have beneficial effects on human health [13]. Increased serum levels of liver enzymes indicate liver injury and ALT is the most important marker of liver injury [14]. The increased liver enzyme levels can be observed alongside simple steatosis in over 50% of the patients, and are observed in approximately 80% of patients with advanced NAFLD [15].
To treat NAFLD patients, it is necessary to use plant-based drugs that cause comparatively fewer side-effects and are available and inexpensive as well as effective in improving liver enzymes, hepatic lipids, and liver morphology with regard to unsatisfactory effects of weight loss and exercise protocols as well as inefficiency, long-term effects and serious side-effects of certain chemical drugs [5,16,17]. Recent studies have highlighted the necessity of conducting further studies for confirming the effects of flavonoids, C. sinensis and its extract on liver injury [1,18].
Although a number of studies have been conducted in different countries, most of them have been conducted on mice [5,12,19,20], and few clinical trials have investigated this issue [21]. This study aimed to investigate the effect of C. sinensis on NAFLD, fatty liver grade, TG and cholesterol levels, the body weight and liver enzymes in children aged 10-16 years.
Materials and Methods
This Quasi-experimental study (single-blind clinical trial) was done in a university endocrine clinic in Shahrekord, southwest of Iran from November 2014 to October 2015. The study was approved by the Ethics Committee of Shahrekord University of Medical Sciences (code: IR.SKUMS.REC.1394.57). The present study population consist of 10-16 years old children. The sample size of this study was estimated 26 people in each group according to a formula of sample size calculation (Equation 1) and taking its parameters into account:
Equation 1:
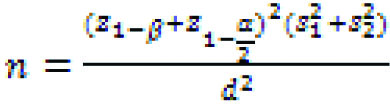
Children with body mass index (BMI) ≥85th percentile or hypertriglyceridaemia (TG >150 mg/dL and/or cholesterol >200 mg/dL) [22] and apparently healthy were examined by a paediatric endocrinologist and gastroenterologist. A detailed history and physical examination was done for all patients. Diagnosis was confirmed by ultrasound and laboratory evidence of fatty liver. The authors selected children with fatty liver on sonography and increased AST and ALT levels after exclusion of other causes. Grading of the fatty liver was conducted according to the protocol described by Saverymuttu SH et al., [23]. All the imaging was done by the same radiologist who was unaware of intervention.
The participants were selected by convenience sampling and were divided into two groups after they provided informed consent to participate in the study and were ensured that the data would be kept private. The present authors used minimisation for randomisation. The data on demographics (age, sex, BMI), the results of ultrasound, ALT, AST, High-Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL), and TG, as well as weight before and after the intervention and any side-effects were recorded in a checklist.
Exclusion criteria were: taking ethanol and hepatotoxic drugs and suffering from cirrhosis of the liver, peptic ulcer, hyperthyroidism, severe anxiety, and severe liver, heart, and renal failure, and systemic diseases.
For both groups, modification of diet and intensification of physical activity were prescribed for three months. Meanwhile, intervention group was treated with green tea 500 mg tablet (Green teadin, Dineh Iran Company.), prepared from pulverised C. sinensis leaf and containing 50 mg total standardised polyphenol, three times per day. The participants were examined monthly for drug side-effects and were followed up for adherence to the drug use. At completion of the 3-month intervention, height, weight, liver enzymes and lipid profile were measured again; ultrasound was conducted again by the sonographer who conducted the baseline sonography, and the data were recorded.
Statistical Analysis
Data analysis was conducted by ANCOVA using SPSS version 19.0 The p-value <0.05: were considered significant.
Results
A total of 52 patients with NAFLD participated in this study. Thirteen girls and thirteen boys were participated in each of the two groups. The age range of the participants was 10-16 years with mean age of 12.53±1.99 years and 11.84±1.53 years in intervention group and control group, respectively. There was no significant difference in age between the two groups (p=0.16). The mean weight of intervention group was 63.76±16.25 kg and that of control group was 57.38±12.74 kg; and the mean height of intervention group was 148.84±8.15 cm and that of control group was 150.255±49.11 cm. There was no significant difference in weight and height between the two groups (p=0.9 and 0.62, respectively).
In the control group, before intervention, 22 children had Grade 1 fatty liver and four children had Grade 2 fatty liver. After intervention, 21 children had Grade 1 fatty liver and five children had Grade 2 fatty liver. In the intervention group, before intervention, 19 children had Grade 1 fatty liver and seven children had Grade 2 fatty liver. After intervention, 10 children had grade 1 fatty liver and no child had Grade 2 fatty liver. In 16 children, fatty liver did not change and their ultrasound results were normal. Changes in fatty liver grade were significant in intervention group and non-significant in control group [Table/Fig-1].
Comparison of fatty liver grade before and after intervention in green tea group and control group.
| Groups | Intervention diet and exercise+green tea | Control diet and exercise |
|---|
| Grade of fatty liver | Number (%) | Number (%) |
|---|
| Before intervention | Grade II | 7 (27%) | 4 (15.4%) |
| Grade I | 19 (73%) | 22 (84.6%) |
| After intervention | Grade II | 0 (0%) | 5 (19.2%) |
| Grade I | 10 (38.5%) | 21 (80.8%) |
| Grade 0 | 16 (61.5%) | 0 (0%) |
| p-value | <0.0001 | 0.59 |
p<0.05: Significant
The levels of TG, HDL, LDL, ALT, AST, weight and BMI were recorded before and after intervention in both groups and mean changes in these variables were estimated. Except for the changes in LDL, the changes in other variables were significant in both groups (p<0.05) [Table/Fig-2].
Comparison of mean difference in studied variables before and after intervention in green tea group and control group.
| Groups | Difference after intervention (Mean±SD) |
|---|
| AST U/L | ALT U/L | LDL mg/dL | HDL mg/dL | Triglyceride mg/dL | Weight Kg | BMI (kg/m2) |
|---|
| Control | 25.03±1.50 | 2.34±2.79 | 2.19±4.57 | -1.64±1.35 | 10.07±8.97 | 1.53±0.42 | 0.06±0.18 |
| Intervention | -4.5±1.34 | -13.07±3.18 | -8.19±7.96 | 2.26±0.25 | -40.76±25.36 | -0.63±0.52 | -1.18±0.26 |
| p-value | <0.0001 | 0.006 | 0.6 | <0.01 | 0.034 | 0.006 | 0.006 |
p<0.05: Significant
Discussion
In this study, the effect of C. sinensis tablets on NAFLD in 52 children aged 10-16 years with NAFLD was investigated. As NAFLD is seen in children over 10 years of age so, this age group was selected [4]. Intervention and control groups were not significantly different in terms of age, sex, weight, and BMI. Because fatty liver grade and lipid profile are associated with these variables, their confounding effects on results were neutralised and therefore the results are more likely to be attributed to the effect of C. sinensis in reducing lipidaemia, weight and BMI as well as improving fatty liver. Because antioxidant and anti-inflammatory properties of certain medicinal plants have already been confirmed and the effects of such plants in preventing steatohepatitis in the cases on high-fat diet are promising [20], The authors were encouraged to investigate the effect of C. sinensis consumption on NAFLD. In this study using C. sinensis tablet could improve fatty liver grade, while in control group, fatty liver did not improve. C. sinensis effect on the liver may be attributed to its antioxidant effect. C. sinensis did not cause significant decrease in LDL levels. Moreover, the changes in serum TG levels displayed significant effect of C. sinensis in decreasing TG levels compared to the effects of either diet or exercise. In addition to antioxidant effects, C. sinensis has also anti-hyperlipidemic effects that are possibly due to inhibitory effect of catechin in C. sinensis on phospholipase A2 that leads to decreased absorption of lipids [6]. C. sinensis has also been reported to have anti-apoptotic, nutrient-reducing, and inflammatory response-modulating effects [24]. Animal studies have shown that consumption of tea and catechin decreases triacylglycerol and serum total cholesterol concentration, prevents fat accumulation in the liver and body and stimulates thermogenesis [21]. In addition, drinking 690 mg catechin-containing beverage for 12 weeks caused significant decrease in the body and subcutaneous fat in Japanese healthy men [25]. In the current study, the changes in serum HDL levels confirmed the significant effect of C. sinensis in increasing HDL levels compared to control group, representing its contribution to weight loss even in the short term. In a study using different doses, daily diet +1.3 mg/g EGCG led to weight loss in DSS-treated BALB/c mice [19]. However, indefinite results regarding the anti-obesity effects of C. sinensis have also been reported [15]. A study showed significant weight loss, decrease in BMI and waist circumference as well as similar decreases in the plasma levels of total cholesterol and LDL after 12 weeks’ treatment with high dose of EGCG in women aged 20-60 years. The anti-obesity mechanism might be, to a certain extent, related to inhibition of ghrelin secretion, which resulted in increased adiponectin [26].
In many studies, decrease in LDL has occurred after treatment with C. sinensis [27-30]. Decreased absorption of cholesterol and liver cholesterol concentration leads to increase in expression and activity of LDL receptors and this increased activity in the liver-specific cells cause cholesterol uptake from the blood flow [28]. In the present study, such decrease was also seen but it was not significant. This inconsistency in the findings can be due to differences in patients and duration of C. sinensis treatment and dose as well as the route of administration.
A study indicated that 24-week treatment with C. sinensis in overweight Japanese children, compared to those followed up on exercise and diet, caused significant decrease in LDL [27]. These children, however, were not NAFLD patients. In this regard, the study of Basu A et al., demonstrated significant decrease in weight and BMI, decrease in LDL and total cholesterol and increase in HDL after eight-week treatment with C. sinensis in obese men and women [30], which is consistent with the present findings regarding weight loss and HDL improvement. Consistent with the current study, Suliburska J et al., reported that supplementation with C. sinensis led to decreased total levels of cholesterol, LDL and triglyceride and increased levels of HDL and cholesterol in obese people [31]. Another study showed that taking C. sinensis caused significant decrease in LDL and remarkable increase in HDL [29]. In addition, eight-week treatment with C. sinensis extract did not cause any significant change in total cholesterol and TG, but resulted in increase in HDL concentration, decrease in adipose and improvement of lipolytic pathways [32]. In a study on diabetic people, one-month consumption of C. sinensis did not lead to significant change in total cholesterol, TGs, HDL, and LDL [33]. GrTPs can decrease the risk of certain complications such as cirrhosis, non-alcoholic steatohepatitis, and hepatic cellular carcinoma [5].
In the present study, the levels of ALT and AST decreased after C. sinensis treatment as compared to control group. This medicinal plant has potential to prevent steatosis and therefore injuries due to oxidative stress, i.e., steatohepatitis. A study with mice reported that consumption of C. sinensis extract alongside exercise decreased plasma ALT level by 92% [34]. Significant decrease was observed in AST and ALP levels in rats orally administered with 10% aqueous C. sinensis extract compared to rats treated with 20% C. sinensis extract while the level of ALT in group fed with 20% of C. sinensis extract decreased significantly [35]. A study on diabetic people reported that only ALT increased significantly after C. sinensis consumption compared to controls. Other liver enzymes and the lipid factors did not change significantly [33]. Besides that, Hasanein AM et al., reported the most prominent decrease in the mean levels of AST, ALT and ALP was observed in the group on high-fat diet and treated daily with aqueous C. sinensis extract [36]. In the study of Pezeshki A et al., C. sinensis extract (500 mg tablet/day) caused significant decrease in ALT and AST compared to placebo in 20 to 50 years-old patients with NAFLD [37], which is consistent with the present results on children aged 10-16 years. The present authors used C. sinensis tablet in the current study, but studies with animals have demonstrated that parenteral administration of C. sinensis is better than taking it orally [29].
The present study showed that C. sinensis compounds exerted protective effects against liver diseases in children and caused decrease in AST and ALT levels and hepatic fat accumulation, improvement of liver function and weight loss. The contribution of phenolic compounds and catechin in C. sinensis to these observations should be taken into account. However, such effects are more prominent when treatment is accompanied by exercise and appropriate diets. Although authors did not observe any side-effects, lack of prescribing polyphenolic compounds in high doses for children should be seriously considered because long-term use of C. sinensis extract in the mice on high-cholesterol diet has led to adverse effects and induced liver injury [9]. Overall, inconsistency in results can be due to differences in duration of C. sinensis treatment, dose, the type and compounds of the tablet or extract used, people’s habitual drinking of C. sinensis in some regions and the form of administration (tablet, syrup, bag, brewed, etc.,).
Limitation
Limitations of the current study was small sample size and short follow-up duration. It is recommended to design future study on larger sample and longer follow-up.
Conclusion
With regard to the positive effects of C. sinensis in decreasing weight, BMI and hepatic fat accumulation as well as improving liver function, decreasing liver enzymes ALT and AST and improving blood lipid profile in children aged 10-16 years in this study, prescription of this medicinal plant, in 500 mg dose, is recommended particularly for preventing development of liver diseases in children especially NAFLD. These effects can be due to certain compounds such as polyphenols especially catechin in C. sinensis and its antioxidant and anti-hyperlipidemic effects.
p<0.05: Significant
p<0.05: Significant
[1]. Shin JH, Jung JH, Non-alcoholic fatty liver disease and flavonoids: Current perspectivesClin Res Hepatol Gastroenterol 2017 41(1):17-24.10.1016/j.clinre.2016.07.00127545758 [Google Scholar] [CrossRef] [PubMed]
[2]. Vernon G, Baranova A, Younossi ZM, Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adultsAliment Pharmacol Ther 2011 34(3):274-85.10.1111/j.1365-2036.2011.04724.x21623852 [Google Scholar] [CrossRef] [PubMed]
[3]. Foroughi M, Maghsoudi Z, Ghiasvand R, Iraj B, Askari G, Effect of vitamin D supplementation on C-reactive protein in patients with nonalcoholic fatty liverInt J Prev Med 2014 5(8):969-75.25489444 [Google Scholar] [PubMed]
[4]. Ludwig J, Viggiano TR, McGill DB, Oh BJ, Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed diseaseMayo Clin Proc 1980 55(7):434-38.7382552 [Google Scholar] [PubMed]
[5]. Tan Y, Kim J, Cheng J, Ong M, Lao WG, Jin XL, Green tea polyphenols ameliorate non-alcoholic fatty liver disease through upregulating AMPK activation in high fat fed Zucker fatty ratsWorld J Gastroenterol 2017 23(21):3805-14.10.3748/wjg.v23.i21.380528638220 [Google Scholar] [CrossRef] [PubMed]
[6]. Sakata R, Nakamura T, Torimura T, Ueno T, Sata M, Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: a double-blind placebo-controlled studyInt J Mol Med 2013 32(5):989-94.10.3892/ijmm.2013.150324065295 [Google Scholar] [CrossRef] [PubMed]
[7]. Song Q, Fresh green tea (in Chinese)Food and Health 2014 02:54-55. [Google Scholar]
[8]. Yin X, Yang J, Li T, Song L, Han T, Yang M, The effect of green tea intake on risk of liver disease: a meta analysisInt J Clin Exp Med 2015 8(6):8339-46.PubMed PMID: 26309486 [Google Scholar] [PubMed]
[9]. Hirsch N, Konstantinov A, Anavi S, Aronis A, Hagay Z, Madar Z, Prolonged feeding with green tea polyphenols exacerbates cholesterol-induced fatty liver disease in miceMolNutr Food Res 2016 60(12):2542-53.10.1002/mnfr.20160022127432221 [Google Scholar] [CrossRef] [PubMed]
[10]. Schneider C, Segre T, Green tea: potential health benefitsAm Fam Physician 2009 79(7):591-94.PubMed PMID: 19378876 [Google Scholar] [PubMed]
[11]. Kim JJ, Tan Y, Xiao L, Sun YL, Qu X, Green tea polyphenol epigallocatechin-3-gallate enhance glycogen synthesis and inhibit lipogenesis in hepatocytesBiomed Res Int 2013 2013:92012810.1155/2013/92012824066304 [Google Scholar] [CrossRef] [PubMed]
[12]. Raederstorff DG, Schlachter MF, Elste V, Weber P, Effect of EGCG on lipid absorption and plasma lipid levels in ratsJ Nutr Biochem 2003 14(6):326-32.10.1016/S0955-2863(03)00054-812873714 [Google Scholar] [CrossRef] [PubMed]
[13]. Chacko SM, Thambi PT, Kuttan R, Nishigaki I, Beneficial effects of green tea: a literature reviewChin Med 2010 5:1310.1186/1749-8546-5-1320370896 [Google Scholar] [CrossRef] [PubMed]
[14]. Nguyen DM, El-Serag HB, The epidemiology of obesityGastroenterol Clin North Am 2010 39(1):1-7.10.1016/j.gtc.2009.12.01420202574 [Google Scholar] [CrossRef] [PubMed]
[15]. Angulo P, Keach JC, Batts KP, Lindor KD, Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitisHepatology 1999 30(6):1356-62.10.1002/hep.51030060410573511 [Google Scholar] [CrossRef] [PubMed]
[16]. Rotman Y, Sanyal AJ, Current and upcoming pharmacotherapy for non-alcoholic fatty liver diseaseGut 2017 66(1):180-90.10.1136/gutjnl-2016-31243127646933 [Google Scholar] [CrossRef] [PubMed]
[17]. Salomone F, Godos J, Zelber-Sagi S, Natural antioxidants for non-alcoholic fatty liver disease: molecular targets and clinical perspectivesLiver Int 2016 36(1):5-20.10.1111/liv.1297526436447 [Google Scholar] [CrossRef] [PubMed]
[18]. Teschke R, Zhang L, Melzer L, Schulze J, Eickhoff A, Green tea extract and the risk of drug-induced liver injuryExpert Opin Drug Metab Toxicol 2014 10(12):1663-76.10.1517/17425255.2014.97101125316200 [Google Scholar] [CrossRef] [PubMed]
[19]. Oz HS, Chen T, de Villiers WJ, Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis modelsFront Immunol 2013 4:13210.3389/fimmu.2013.0013223761791 [Google Scholar] [CrossRef] [PubMed]
[20]. Yao H, Qiao YJ, Zhao YL, Tao XF, Xu LN, Yin LH, Herbal medicines and nonalcoholic fatty liver diseaseWorld J Gastroenterol 2016 22(30):6890-95.10.3748/wjg.v22.i30.689027570425 [Google Scholar] [CrossRef] [PubMed]
[21]. Monteiro R, Assuncao M, Andrade JP, Neves D, Calhau C, Azevedo I, Chronic green tea consumption decreases body mass, induces aromatase expression, and changes proliferation and apoptosis in adult male rat adipose tissueJ Nutr 2008 138(11):2156-63.10.1093/jn/138.11.215618936213 [Google Scholar] [CrossRef] [PubMed]
[22]. Ichimaru N, Takahara S, Kokado Y, Wang JD, Hatori M, Kameoka H, Changes in lipid metabolism and effect of simvastatin in renal transplant recipients induced by cyclosporine or tacrolimusAtherosclerosis 2001 158(2):417-23.10.1016/S0021-9150(01)00438-511583721 [Google Scholar] [CrossRef] [PubMed]
[23]. Saverymuttu SH, Joseph AE, Maxwell JD, Ultrasound scanning in the detection of hepatic fibrosis and steatosisBr Med J (Clin Res Ed) 1986 292(6512):13-15.10.1136/bmj.292.6512.133080046 [Google Scholar] [CrossRef] [PubMed]
[24]. Rameshrad M, Razavi BM, Hosseinzadeh H, Protective effects of green tea and its main constituents against natural and chemical toxins: A comprehensive reviewFood Chem Toxicol 2017 100:115-37.10.1016/j.fct.2016.11.03527915048 [Google Scholar] [CrossRef] [PubMed]
[25]. Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in menAm J Clin Nutr 2005 81(1):122-29.10.1093/ajcn/81.1.12215640470 [Google Scholar] [CrossRef] [PubMed]
[26]. Chen IJ, Liu CY, Chiu JP, Hsu CH, Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trialClin Nutr 2016 35(3):592-99.10.1016/j.clnu.2015.05.00326093535 [Google Scholar] [CrossRef] [PubMed]
[27]. Matsuyama T, Tanaka Y, Kamimaki I, Nagao T, Tokimitsu I, Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in childrenObesity (Silver Spring) 2008 16(6):1338-48.10.1038/oby.2008.6018356827 [Google Scholar] [CrossRef] [PubMed]
[28]. Bursill CA, Roach PD, A green tea catechin extract upregulates the hepatic low-density lipoprotein receptor in ratsLipids 2007 42(7):621-27.10.1007/s11745-007-3077-x17582541 [Google Scholar] [CrossRef] [PubMed]
[29]. Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P, Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trialClin Nutr 2008 27(3):363-70.10.1016/j.clnu.2008.03.00718468736 [Google Scholar] [CrossRef] [PubMed]
[30]. Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndromeJ Am Coll Nutr 2010 29(1):31-40.10.1080/07315724.2010.1071981420595643 [Google Scholar] [CrossRef] [PubMed]
[31]. Suliburska J, Bogdanski P, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A, Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patientsBiol Trace Elem Res 2012 149(3):315-22.10.1007/s12011-012-9448-z22581111 [Google Scholar] [CrossRef] [PubMed]
[32]. Cunha CA, Lira FS, Rosa Neto JC, Pimentel GD, Souza GI, da Silva CM, Green tea extract supplementation induces the lipolytic pathway, attenuates obesity, and reduces low-grade inflammation in mice fed a high-fat dietMediators Inflamm 2013 2013:63547010.1155/2013/63547023431242 [Google Scholar] [CrossRef] [PubMed]
[33]. Khatun MA, Prodhan UK, Rahman N, Acute effects of green tea (Camellia sinensis) intake instead of anti-diabetic drug on hepatic enzymes and atherogenic risk factors in type 2 diabetic patientsInt J Adv Res Biol Sci 2017 4(3):172-78.10.22192/ijarbs.2017.04.03.020 [Google Scholar] [CrossRef]
[34]. Khoo WY, Huang SW, Chrisfield BJ, Sae-tan S, Lambert JD, Decaffeinated green tea and voluntary exercise prevent non-alcoholic fatty liver disease in miceFASEB J 2017 31(1 Supplement):458-52. [Google Scholar]
[35]. Bakr ES, Header EA, Effect of aqueous extract of green tea (Camellia Sinensis L.) on obesity and liver status in experimental ratsInt J Pure Appl Sci Technol 2014 22(1):53-63. [Google Scholar]
[36]. Hasanein AM, Gawad HS, El-Megeid AA, Effect of water extract prepared from green tea, black tea and cinnamon on obese rats suffering from diabetesWorld Appl Sci J 2012 20(7):976-87. [Google Scholar]
[37]. Pezeshki A, Safi S, Feizi A, Askari G, Karami F, The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver diseaseInt J Prev Med 2016 7:2810.4103/2008-7802.17305126955458 [Google Scholar] [CrossRef] [PubMed]