The cardiovascular disease factor start early in age and the risk factors tend to track and magnify with age. The risk factors of cardiovascular diseases are considered to be smoking, obesity, diabetes, hypertension, glucose intolerance and lipid abnormalities. In high income countries, the main risk factor is obesity for below the age of 70 and in low/middle income countries, it is smoking. Obesity may cause high blood pressure, lipid abnormalities, diabetes, and metabolic syndrome by aging. Thus, there is a higher incidence of death below the age of 70 in low/middle income countries because of lower obesity incidence and higher smoking incidence [1]. The death rate for coronary heart disease is 157.11 per 100,000 of Turkish population which occurs as 25.37% of total deaths [2]. Previously, it was indicated that low levels of total cholesterol and High-Density Lipoprotein-Cholesterol (HDL-C) is a precursor of cardiovascular diseases in Turkish population when comparing with other European populations [3-6]. Therefore, figuring out possible etiologic factors is crucial in order to diagnose and treat this disease.
The atherosclerotic process begins with the disruption of the endothelial lining of an artery. As part of an inflammatory response to the damaged artery, platelet-derived growth factors pass through the hyperpermeable vessel lining causing proliferation of smooth muscle, an integral part of the vessel wall. Lipoproteins in the bloodstream become lodged in the vessel wall where damage has occurred, further adding size to the developing plaque. This thickened and raised lesion protrudes into the vessel lumen altering blood flow and may cause embolism, thrombosis and so ischemia [7]. The calcifications in vessels which supplies the brain, are risk factors for dementia and stroke [8]. Atherosclerosis is a systemic vascular process which can lead to several diseases including cardiovascular and cerebrovascular disease [9]. Angina pectoris, myocardial infarction, arrhythmia and congestive heart failure are most important cardiovascular diseases because of atherosclerosis, and as cerebrovascular disease, ischemic stroke usually results from atherosclerotic plaques. One of the common locations for atherosclerosis are bilateral common carotid arteries which ascend in the neck and at the level of the superior border of the thyroid cartilage, the other sites are abdominal aorta, coronary arteries, and popliteal arteries [7].
Previously, periodontal disease was named to be a risk factor for stroke depending coronary heart disease. Previous studies demonstrated that severe periodontal disease may cause cardiovascular problems such as stroke [10-13].
Chronic Periodontitis (CP) is distinguished as loss of periodontal attachment and alveolar bone [13-19]. Previous studies demonstrated microorganisms related to periodontal diseases in carotid endarterectomy which establishes, an association between chronic periodontitis and carotid atherosclerosis [20-24].
Early identification and understanding of behavioral and physiological variables and risk factors related to heart diseases such as “periodontal status of the patients” are essential. Previous studies [7, 25-29] have demonstrated relationship between periodontitis and cardiovascular disease by using panoramic radiograph. In panoramic radiographs, CAC may appear as nodular radiopaque masses or radiopaque vertical lines inferior or posterior to the angle of mandible [Table/Fig-1a]. Panoramic reconstructed CBCT image showing alveolar bone loss is depicted in [Table/Fig-1b].
CAC must be distinguished from other radiopacities in this area such as the hyoid bone, epiglottis, stylohyoid ligament, submandibular gland sialolith, phleboliths, triticeous cartilage of the thyroid ligament, superior cornu of the thyroid cartilage, and cervical lymph nodes. Because of two dimension nature, CAC may misdiagnosed in panoramic radiographs. CBCT is a more accurate method for diagnosing CAC by determining exact locations of calcifications. Also, panoramic radiograph is not a suitable image modality for diagnosing small and less calcified carotid calcifications accurately, compared with CBCT. In the present study, evaluation of carotid calcification have demonstrated by using CBCT images and so, relationship between periodontitis and carotid calcification have demonstrated more accurately. The presence of such calcifications especially large and elastic arteries was found to occur more predominantly [30].
Therefore, the aim of this current research was two folded: (1) to figure out the prevalence of CAC using CBCT images with a FOV including entire craniofacial area; and (2) to examine the correlation between CAC and radiographic evidence of alveolar bone loss.
Materials and Methods
This retrospective study was done in Ankara University, Department of Dentomaxillofacial Radiology from January 2014 to August 2017. The CBCT records of 256 patients were archived in this study. The CBCT in these patients were done for various indications such as temporomandibular dysfunction, evaluation of paranasal sinuses, trauma and orthognathic surgery planning. There was no specific population or patient in this study. For this evaluation, this study included as many patients’ CBCT images as we can find from the archieve that fits the selection criteria in the study according to the “Law of Large Numbers”. The primary inclusion criteria were full Field of View (FOV) of CBCT examinations which included entire craniofacial area. The exclusion criteria were made according to patients’ medical records. Patients with any systemic diseases were excluded from the study and, also evidence of bone disease such as osteoporosis, or any surgical investigations due to pathological conditions in maxilla and mandible as well as syndromic patients were excluded. Also, age under 35 were excluded because of limited number and smaller FOV. During evaluation of CBCT images, edentulous patients were excluded which were not suitable for this study. After exclusion, remaining number of patients were 256. After evaluation of 256 patients’ CBCT images, 60 patients with CAC were found. These 60 patients were chosen as experimental group and classified according to age into three groups as: <50, 50-59, >59 years of ages. The age ranges were chosen by distribution of ages of patient with CAC. Additionally, in remaining 196 patients, 60 patients were chosen randomly for control group.
Ethical approval was obtained from the Human Research Ethics Committee of our institution (ethical approved number: 36290600/01). There was no preference for gender regarding sample choice.
Imaging using CBCT
CBCT scans (Planmeca 3D MAX (Planmeca, Helsinki, Finland) which included the craniofacial anatomy, were chosen. CBCT exposures were made in 96 kVp and 12 mA at 0.400-mm3 voxel size. The FOV was 23 cm in diameter and 26 cm in height with a full head reconstruction. Since CBCT machines acquires acquisition in cone shaped area, the entire field of view including neck and skull can be made in a single scanning.
Axial, sagittal and 3D reconstructions were reconstructed for all skulls with 1024×1024 pixel slices. All visualisations were done using a medical monitor.
Image Evaluation
A periodontologist and an oral and maxillofacial radiologist (NB, US) with an experience of 10 years and 2 years respectively, who were blinded to clinical status of the patients, evaluated CBCT scans respectively. The observers were allowed to use the enhancement tools of the software. The images were evaluated in a 2-week interval, and all investigations were repeated after 2 months. Interobserver and intraobserver agreement were calculated.
All CBCT images (N=256) were evaluated for the presence of CAC as nodular radiopaque masses or radiopaque vertical lines adjacent to the cervical vertebrae C3, C4, or the intervertebral space between them [Table/Fig-2]. CAC was detected in 60 patients. To make a cross correlation from the findings of CAC with radiographic evidence of alveolar bone loss, an additional out of 196 healthy subjects, 60 were randomly selected as control groups (age range 35-70 year; 26 females-34 males; mean age 44.3±10.5 years) (age and gender-match, free of systemic disease).
Same patient’s: a) coronal; b) sagittal; c) axial; and d) 3D reconstructed slices showing bilateral CAC anterior C3-C4.
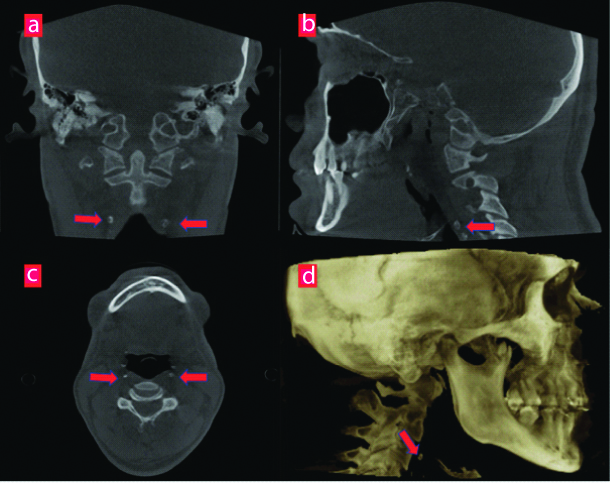
Using retrospective data from current study, a power analysis (Power and Precision software, Biostat, Englewood, NJ, USA) was conducted that indicated that detection of differences between groups could be obtained with at least 50 patients at a power of 0.95 (alpha=0.55). Thus, this final study was conducted as 60 patients with and without CAC using CBCT images which was along with the power analysis.
Of all the new study group, alveolar bone loss measurements were made on all teeth including maxilla and mandible via machine’s dedicated software [Table/Fig-1b].
Percent alveolar bone loss was calculated using the scoring criteria devised and reported by Craft G et al., [31]. PRI is an index to calculating the percent of alveolar bone loss in a patient. This index gives an overall rate for alveolar bone loss for all regions of maxilla and mandible and make an overall percent of alveolar bone of an individual rather than calculation only one region or tooth. The alveolar crest was considered to be that point at which the periodontal ligament maintained a normal width. The normal height of the alveolar crest was defined as 1 mm apical to CEJ [Table/Fig-3]. The radiographic root length of all teeth was measured from the CEJ to the root tip.
a,b) Panoramic reconstructed CBCT image showing alveolar bone loss measurements.
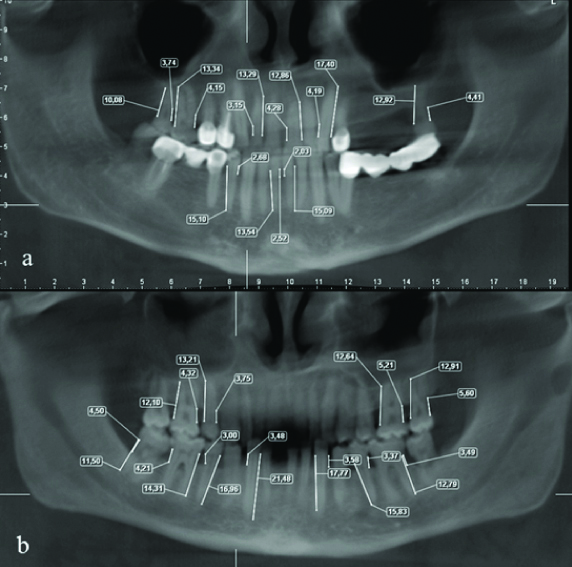
The index is based on measuring the radiographic root length and also the radiographic alveolar bone loss and then estimate the alveolar bone loss of teeth by diving the distance from the CEJ to the alveolar crest minus 1 with the root length and multiplied by 100. Total bone loss was calculated in a similar manner by adding the individual values from a given subject then dividing the total by the number of teeth.
Statistical Analysis
Statistical analyses were carried out using the SPSS 22.0 software (SPSS, Chicago, IL, USA). Intra and inter-examiner validation measures were conducted. To assess intra-observer reliability, the Wilcoxon matched-pairs signed rank test was used for repeat measurements.
Cohen’s Kappa coefficient (κ) was calculated for assessing the inter-examiner agreement between observers using the NCSS 2007 statistical software (NCSS and GESS, NCSS, LLC. Kaysville, UT, USA) using the guidelines proposed by Bland JM et al., the strength of agreement between 0 and 1 can be interpreted as follows: 0.00-0.20 slight agreement, 0.21-0.40 fair agreement, 0.41-0.60 moderate agreement, 0.61-0.80 substantial agreement, 0.81-1.00 almost perfect agreement [32]. A κ value that reaches 0.40 or better is considered to be acceptable for clinical use.
Moreover, the periodontal bone loss and age variables normality test were done using Kolmogorov-Smirnov test. The variables were not disturbed normally, then groups were compared using Mann-Whitney U test. Comparison of Alveolar Bone loss according to CAC (Unilateral/Bilateral) was statistically evaluated using Pearson chi-square test (p<0.05).
Results
Intra-Observer Consistency
The observers made 1st and 2nd reading with a two month interval. The intra-observer kappa coefficients for 1st and 2nd readings of the observers ranged from κ=0.880 to κ=0.920 which indicated high agreement between observers for the first and second readings. The analysis demonstrated that the procedure was standardised between the evaluations and measurements of the observers. No statistical differences were found among observers’ evaluations and measurements (p>0.05). The observer 1 had highest intra-observer consistency, thus the mean of this observers evaluation and measurements were chosen for further analysis of alveolar bone loss.
Inter-Observer Consistency
There was no significant inter-observer difference for both observers in repeated CBCT evaluation and measurements (p>0.05). Overall intra-observer consistency for observer 1 was rated at 90.4% and 95.1%, while the consistency for observer 2 was found 84.6% and 89.6% between the two evaluations and measurements, respectively. All measurements were found to be highly reproducible for both observers and no significant difference was obtained from two measurements of the observers (p>0.05).
Age Estimation
The overall mean age was 46.5 years. The age was 35-84 years with a standard deviation of 15.4 years. The mean age of the male patients was 46.5 with a standard deviation of 14.1 (range, 35-72 years), while 44 years for females with a standard deviation of 14.1 (range, 36-84 years)
CAC Prevalence According to Age and Gender
In total 60 patients (age range 35-70 year; 25 females-35 males; mean age 44.5±9.69 years) had CAC (23.4%) [Table/Fig-4]. Of the 139 male patients, 104 (74.8%) had no calcifications, 26 (18.7%) had bilateral CAC and 9 (6.5%) had unilateral CAC. Of the 117 female patients, 92 (78.7%) had no detectable calcifications. Twenty (17.1%) had bilateral CAC while 5 (4.3 %) had unilateral CAC. Of the 136 subjects younger than 50 years, 93 (68.4%) had no CAC. In total 43 CAC detected in the patients younger than 50 years. Thirty-one (22.8%) had bilateral calcifications, 12 (8.8%) had unilateral CAC. Of the 68 subjects 50 to 59 years, 59 (86.8%) had no detectable CAC while, 8 (11.8%) had bilateral and 1 patient (1.5%) had unilateral CAC. Of the patients 52 patients 60 to 70 years, 44 (84.6%) had no CAC while 7 (13.5%) had bilateral and 1 patient (1.9%) had unilateral CAC.
Distribution of patients with CAC according to age and gender.
| <50 | 50-59 | 60-70 | Total |
|---|
| Male | 24 | 7 | 4 | 35 |
| Female | 19 | 2 | 4 | 25 |
| Total | 43 | 9 | 8 | 60 |
Of all CBCT images, in total 60 patients (age range 35-70 year; 25 females-35 males; mean age 44.5±9.69 years) had CAC (23.4%). CAC in present study were detected in 35 (58.3%) males and 25 (41.7%) females. Significant difference was found for male patients than females (p >0.05). In total 46 patients had bilateral calcifications (38.33%) and 14 patients had unilateral calcification (11.66%) in the bifurcation area.
No calcification was visible on the other CBCT images for 196 (76.6%). Alveolar bone loss was evaluated in 60 patients with CAC and 60 age and gender-matched patients with free of systemic diseases.
CAC and Alveolar Bone Loss according to Gender and Age
Males with CAC had a mean bone loss of 16.8%±14.1, while females with CAC had a mean bone loss 15.6%±14.7 [Table/Fig-5]. No significant difference was found between male and female patients according to control group (p=0.853).
Comparison of study group (patients with CAC)/control group (patients without CAC) and alveolar bone loss according to gender with student t-test.
| Gender | n | Bone loss % | SD | p-value |
|---|
| Study group | Male | 35 | 16.8 | ±14.1 | 0.853 |
| Female | 25 | 15.6 | ±14.7 |
| Control group | Male | 35 | 10.4 | ±10.1 | 0.836 |
| Female | 25 | 8.5 | ±7.9 |
p-value less than 0.05 shows statistical significance; n: number of CAC; SD: Standard deviation
Patient younger than 50 years had a mean percent of bone loss of 13.3%±12.6. Those aged 50 to 59 years had a mean bone loss of 19.9%±14.7. Those aged 60 to 70 years 28.4%±15.8. While bone loss did increase considerably according to age groups, statistical significant difference was found between group younger than 50 and 60-70 age group [Table/Fig-6].
Comparison of study group (patients with CAC)/control group (patients without CAC) and alveolar bone loss according to age with student t-test.*p-value less than 0.05 shows statistical significance.
| Age | n | Bone loss % | SD | Pairwise comparisons | p-value |
|---|
| Study group | <50 (1) | 43 | 13.3 | ±12.6 | 1-2 | 0.364 |
| 50-59 (2) | 9 | 19.9 | ±14.7 | 2-3 | 0.218 |
| >59 (3) | 8 | 28.4 | ±15.8 | 1-3* | 0.022* |
| Control group | <50 (1) | 41 | 7.4 | ±9.1 | 1-2* | 0.042* |
| 50-59 (2) | 13 | 14.4 | ±7.7 | 1-3* | 0.048* |
| >59 (3) | 6 | 14.0 | ±8.2 | 2-3 | 0.069 |
Student t-test; *p-value less than 0.05 shows statistical significance; n: number; SD: Standard deviation
Control Group Alveolar Bone Loss According to Gender and Age. In control group, males had a mean bone loss of 10.4%±10.1, while females had a mean bone loss 8.5%±7.9. No significant difference was found between male and female patients according to control group (p=0.836) [Table/Fig-5].
In the control groups, patient younger than 50 years had a mean percent of bone loss of 7.4%±9.1. Those aged 50 to 59 years had a mean bone loss of 14.4%±7.7. Those aged 60 to 70 years 14.0%±8.2. While bone loss did increase considerably according to age groups, statistical significant difference was found between group younger than 50 and the others [Table/Fig-6].
CAC/Control- Alveolar Bone Loss Statistical Significance
There was highly significant correlation between CAC and percent of alveolar bone loss. The patients with CAC had higher alveolar bone loss than the control subjects according to all age groups and gender (p<0.05). Radiographs showing unilateral and bilateral CAC had a mean percent bone loss of 9.7±7.6%, 18.6±14.9%. The patients with bilateral calcifications had higher prevalence of alveolar bone loss (p<0.05). The analysis showed that the 2 variables are correlated. The strength of this association is represented by the correlation coefficient r=0.9460, and it is considered a strong positive association. (p=0.024) [Table/Fig-7].
Distribution of Alveolar Bone loss according to CAC (Unilateral/Bilateral).
| CAC | n | Bone loss % | SD | Pearson-Chi-square test |
|---|
| Bilateral CAC | 46 | 18.6 | ±14.9 | 0.024* |
| Unilateral CAC | 14 | 9.7 | ±7.6 |
Pearson chi square test *p-value less than 0.05 shows statistical significance; CAC: Carotid artery calcification; n: Number; SD: Standard deviation
Discussion
In this study, CBCT was used in order to detect alveolar bone loss and CAC. It is difficult to determine the actual degree of alveolar bone loss in two dimensional images due to image overlapping [33]. Recent studies indicated that CBCT is reliable for evaluating periodontal defects and bone loss [34,35]. Also, there are few studies using CBCT images for detecting relationship between alveolar bone loss and carotid arterial calcifications. Therefore, CBCT images were preferred for this study.
Tamura T et al., indicated a significant higher prevalence for females [36], whereas Beckstrom BW et al., found a male predilection for CAC [7]. The relationship between calcifications and periodontal disease is still obscure, since periodontal infections were constituted with higher inflammatory markers such as tumor necrosis factor alpha and interleukin-1 beta which shows the peripheral reaction that can accelerate the progression of pre-existing atherosclerotic plaques [37]. Hence, its possible contribution to vascular disease is of potential importance.
The present study show that carotid arteria calcifications were more located on both sides (76.7%) with a slightly greater prevalence on the right side. Fourteen unilateral calcifications were detected in this study, and 10 were located in the right side and 4 in the left side. This could be due to the small sample of in this study; however results provided from previous studies describe a right prevalence tendency of CAC 13.34% while another study reports higher prevalence of the left localization of carotid artery calcifications [38]. This may be again due to the population characteristics.
One of the main causes for vascular disease may start with the formation of atherosclerotic process. Periodontal pathogens (Porphyromonasgingivalis, Aggregatibacter actinomycetemcommitans, Treponema denticola, Prevotella intermedia and Tannerella forsythia), could play a role in the development and progression of atherosclerosis [7]. D’Aiuto F et al., studied the correlation between periodontitis and atherogenesis and stated that severe generalised periodontitis causes systemic inflammation [23]. This is consistent with a causative role of periodontitis in atherogenesis.
In a study by Engebretson SP et al., concluded that the presence of carotid artery plaques may increase in patients with severe periodontal disease [24]. Ravon NA et al., reported that patients with CAC detected by ultrasound were more likely to have more teeth with over 5 mm of alveolar bone loss measured on a panoramic radiograph than those negative for carotid artery atherosclerosis [21]. Our study is in agreement with these previous studies that showed significant relationship between the presence of carotid artery calcifications and increased percent alveolar bone loss. However, Ohba T et al., also reported no relationship between CAC and Community Periodontal Index (CPI) [38]. In our study, we found alveolar bone loss higher in patients with CAC than the control subjects without any systemic diseases which supports to previous research stating that periodonthopathic bacteria (Porphyromonasgingivalis, Aggregatibacter actinomycetemcommitans, Treponema denticola, Prevotella intermedia and Tannerella forsythia), can trigger an immune response associated with atherosclerosis [39]. In this study a prevalence of 23.4% was found for CAC in a cross-section study group. This finding is in line with the results of Beckstrom BW et al., but co-incidence from Chen J et al., study [7,40]. The reason for such difference may due to either population or the method of the study.
CAC in our study were detected in 35 (58.3%) males and 25 (41.7%) females. Significant difference was found for male patients than females (p >0.05). In previous studies by Ohba T et al., Gender, age, diabetes, HDL were associated with heart disease [38]. Another major risk factor for both CAD and periodontal disease is smoking with its potential pathogenic properties [3-5]. According to Onat A [5] smoking is an important factor among adults which may lead to coronary mortality, with significance in men. These findings may justify the higher male predominance for CAC presence in this study.
Limitation
Because of retrospective nature of this study, periodontal status of patients was evaluated without intraoral examination. Although CBCT images have demonstrated alveolar bone loss, accurate periodontal status of patients was uncertain. Determining periodontal status with only alveolar bone loss on CBCT images was most important limitation of this study. And also, age groups were selected according to age distribution of patients with CAC. So age gaps were not equal. To overcome this limitation, close number of patients were selected for each age group in control group.
Conclusion
The null hypothesis was accepted as the alveolar bone loss increase with the prevalence of carotid calcification. Radiographic parameters revealed that alveolar bone loss was more prevalent among subjects with carotid artery calcifications. The study also suggests understanding the interactions of periodontal diseases and CAC can help to focus the public health effort needed to change these patterns. Periodontal status and its role as a risk factor in triggering cardiovascular events should be taken into account by not only dental professionals but also the medical professionals in order to plan the prevention programs.