Introduction
COPD is an irreversible disease which is characterised by progressive airflow obstruction due to inflammatory response of the lung and respiratory passage on exposure to noxious particles [1]. COPD is a common non-communicable disease that contributes to morbidity and mortality among respiratory disease all over the world. According to the study done on Global Burden of Disease, it was estimated that COPD will be the third leading cause of death among non-communicable disease by 2020 [2,3].
Conventionally, the tool used to diagnose stable COPD is the forced expiratory volume in the first second of expiration (FEV1). Owing to lack of available diagnostic laboratory tests, Acute Exacerbation- COPD (AE-COPD) are often diagnosed based on clinical symptoms, which is subjective and is prone to inter-observer variations.
Therefore, there is a need for a better tool for the diagnosis of COPD and to monitor its prognosis. Various biomarkers like CRP, suPAR have been used for COPD diagnosis.
The assessment of suPAR level is being used clinically not only to diagnose COPD but also to diagnose other clinical conditions like Congestive Heart Failure (CHF), Systemic Inflammatory Response Syndrome (SIRS) and Adult Respiratory Distress Syndrome (ARDS). The levels of suPAR vary among AE-COPD, stable COPD and normal controls. The prognostic value of suPAR level among COPD patients may aid in the early diagnosis of underlying pulmonary low grade inflammation, thus allowing the physicians to provide targeted therapies and avoiding unnecessary side-effects of prolonged exposure to drugs, and also avoiding incomplete treatment of COPD [4].
In this review, authors discuss the available literature on suPAR in COPD and provide a descriptive overview of diagnostic and prognostic role of suPAR in COPD.
Discussion
Pathophysiology of COPD
In a normal individual, the damaged respiratory epithelial cells are removed by alveolar macrophages. Inflammatory cytokines like colony stimulating factors and growth factors helps to dampen inflammation, thereby promoting lung repair. However, in COPD patients due to chronic exposure to smoke and dust particles, the inflammatory cells recruitment occur along the respiratory passage and alveoli of the lung. These inflammatory cells release proteinases that damage the extracellular matrix of the lung resulting in inflammation followed by remodelling and scarring of damaged tissue which cause narrowing of the small airways. Moreover, if the exposure to the irritant persists, the bronchial wall becomes inflamed and pus starts to accumulate in the airway lumen [5].
Role of the Immune System in COPD
COPD is most commonly associated with intrinsic immune system disturbances in the lung. Alteration in the number and function of lymphocyte and macrophages has been observed among COPD patients [6]. Due to defect in phagocytic activity of alveolar macrophages the digestion of apoptotic alveolar epithelium gets altered [7]. Moreover, abnormal alteration in polymorphonuclear cells functions and cytokine production with suppression of CD19, CD31, CD44 and CD71 among COPD patients, alters the innate immunity which leads to recurrence of bacterial infection with acute exacerbation [8-10].
Structure and Function of suPAR
The urokinase plasminogen activator/inhibitor system consists of a Plasminogen Activator Inhibitor (PA-I), the receptor for Urokinase Plasminogen Activator (uPAR) and an inactive protease. The uPAR is expressed on the respiratory epithelial cells, neutrophils and macrophages. In 1991, the active form of uPAR called suPAR was identified in serum. uPAR is a G-protein coupled receptor with three domains named Domain I (DI), Domain II (DII) and Domain III (DIII) [Table/Fig-1], information collected from literature [11-13]. Domain-III has chemotactic property for activating the immune system. When uPAR gets activated by a trigger stimulus, the suPAR gets detached from the cell membrane into the blood and other body fluids [14].
Structure of suPAR and its role in inflammation [11-13].
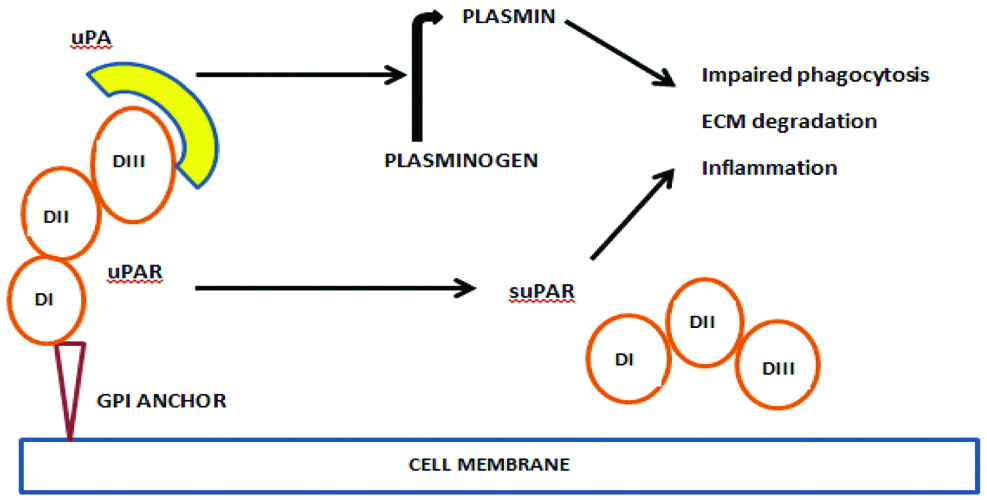
suPAR activates plasminogen to plasmin which causes breakdown of proteins in the extracellular matrix during invasion of foreign body or pathogens [15-19]. It also modulates integrin function during chemotaxis, cell adhesion and intracellular signalling. Hence, suPAR contributes to cell proliferation, inflammation, immune system activation, tissue remodelling and signal transduction [20-23].
The level of suPAR in blood, serum and cerebrospinal fluid correlates proportionately with the activation of the immune system. Elevated levels of serum suPAR are associated with adverse prognosis, such as the possibility of developing sepsis in emergency ward patients and in systemic inflammatory response syndrome (SIRS). The level of suPAR measured in the serum, cerebrospinal fluid and urine are constant throughout the day, with limited circadian rhythm compared to the other inflammatory biomarkers like Interleukin and CRP. Hence, suPAR has been used as a marker clinically for the diagnosis of these diseases [17,24-26]. The serum level of suPAR can be measured by routine double sandwich Enzymelinked Immunosorbent Assay (ELISA) technique using commercial kits [13].
Clinical Significance of Serum suPAR
Clinically, suPAR has been used as an independent indicator of mortality among congestive heart failure patients [27]. In Intensive Critical Care (ICU) settings, the distinct advantage of suPAR in addition to APACHE and SOFA score in predicting the outcome of patients with adult respiratory distress has been documented [28,29].
Diagnostic and Prognostic Value of suPAR in COPD
The importance of early prediction of exacerbation among COPD patients cannot be understated as it helps in the ideal management of the disease [30]. Efficient and early intervention in case of exacerbation in patients with COPD are important strategies in the prevention of mortality in these patients. Hence, a “biomarker” is required that could predict the underlying low grade pulmonary inflammation, that can lead to these exacerbations. An ideal biomarker should diagnose the disease severity, exacerbations and predict the mortality [31]. Traditionally, mainly CRP and fibrinogen have been used as biomarkers to determine COPD exacerbation and response. suPAR estimation has been found to have a diagnostic and prognostic role in sepsis and AE-COPD [32]. In a COPD clinic, an ideal biomarker is expected to facilitate the diagnosis of COPD and to predict the prognosis after treatment [33].
suPAR is a novel biomarker of low-grade pulmonary inflammation which is usually associated with the pathogenesis of lung disease. uPA system has a significant role in the development of COPD by activating the low-grade pulmonary inflammation, lung parenchymal destruction and small airway fibrosis that lead to decline in lung function. As a potential biologically stable marker, suPAR is being used clinically to predict the prognosis for sepsis, congestive heart failure and ARDS [34].
A prospective study done by Gumus A et al., on 43 patients with acute exacerbation COPD (AE-COPD) and on 30 healthy controls showed that the median plasma suPAR value was significantly higher among AE-COPD patients than in controls. After treating AE-COPD patients there was a statistically significant decrease in the median value of suPAR (p<0.001) [35].
Another prospective study by AboEl MGH and Mabrouk MM, showed that suPAR was high among 45 AE-COPD patients than in the 20 controls. Serum levels of suPAR and fibrinogen were measured among different grades of COPD patients on day 1 and day 14. It was observed that serum biomarkers were more on day 1 than post-treatment. There was a positive correlation (r=0.715) between the serum suPAR level and fibrinogen which was statistically significant (p<0.001) [36]. Previous studies on diagnostic and prognostic value of suPAR among COPD patients are summarised in [Table/Fig-2] [35-43].
Summary of the previous studies on the diagnostic and prognostic value of suPAR among COPD patients.
| Author/Year/Reference no. | Study population | Study method | Findings |
|---|
| Xia W et al., 2005 [39] | COPD:74Asthma:34Control:44 | Cross-sectional | Sputum suPAR level was high among COPD (583±871 pg/mL) compared to asthma (399±103 pg/mL) and control (85±11 pg/mL) |
| Gumus A et al., 2015 [35] | AE-COPD: 43Healthy control:30 | Prospective | 1. Median value of suPAR for control: 2.36±0.89 ng/mLAE-COPD on Day 1: 3.38±1.34 ng/mLAE-COPD on Day 7: 4.84±1.902. suPAR plasma levels was found to be negatively correlated with FEV1 post-bronchodilator (p=0.001, r=-0.478 |
| Kurtipek E et al., 2015 [37] | Stable COPD:54AE-COPD:53Post treatment COPD:52 | Prospective | suPAR level during AE-COPD was 1.28±0.52 ng/mL compared stable COPD 1.21±0.59 ng/mL with p=0.49. The level of suPAR reduced by 1.20±0.41 ng/mL after treatment |
| Can U et al., 2015 [38] | Stable COPD:46Control: 41 | Cross-sectional | suPAR level was high in stable COPD 4.94±2.79 than control 2.40±2.01 ng/mL |
| AboEl MGH et al., 2018 [36] | 45-AECOPD20-controls | Prospective | suPAR value was high among AE-COPD patient than controls. The level of suPAR was reduced from day 1 (4678.6±1478.9 pg/mL) on day 1 (3521.3±1382 pg/mL) on day 14 (p<0.001) |
| Godtfredsen NS., 2018 [41] | AE-COPD patients- 2838 | Retrospective | suPAR level was higher among AE-COPD who died within 30 days Vs. than who survived: 5.7 ng/mL (IQR 3.8-8.1) Vs. 3.6 ng/mL (IQr 2.7-5.1) with p<0.0001 and Hazard ratio was 2.0 (95% CI 1.7-2.4) |
| Bocskei RM., 2019 [42] | COPD patients-24Control-18 | Cross-sectional | Plasma level of suPAR was elevated in COPD patients (2.84±0.67 ng/mL) than controls (2.41±0.57 ng/mL) with p=0.03 |
| Loukeri A., 2016 [43] | Stable COPD patients-9Control- | Cross-sectional | Level of suPAR was high among COPD Vs. controls (median value 3.3 ng/mL Vs. 2.5 ng/mL) with p<0.001. |
The study done by Kurtipek E et al., showed that the suPAR level was more among AE-COPD than Stable COPD patients. In addition, the post-treatment suPAR levels among AE-COPD patients were lower compared to pre-treatment levels [37].
Can U et al., measured suPAR levels among 46 stable COPD patients and reported increased level of suPAR among COPD patients possibly due to underlying pulmonary inflammation [38]. Xiao W et al., has found that suPAR level among COPD patient was significantly higher than asthma and controls. This helps to discriminate COPD with other lung pathology. On the whole, suPAR can be used to diagnose AE-COPD and to monitor treatment response [39].
In a study done on 84 stable COPD patients and 51 healthy subjects, to investigate if PA-I was involved in COPD pathogenesis, the serum level of PA-I was significantly increased among stable COPD patients 125.56±51.74 ng/mL (mean±SD) versus the healthy subjects 102.98±36.62 ng/mL (mean±SD). In addition, the level of PA-I showed statistically significant negative correlation (r=-0.308) with pulmonary function parameters (p<0.001) [40].
The diagnostic and prognostic importance of suPAR among sepsis patients and in AE-COPD patients has been reviewed in this paper. However, the contribution of suPAR to predict ongoing low grade pulmonary inflammation has not been dealt with and can be of use if reviewed.
Conclusion
Serum suPAR is a clinically validated biomarker which can help in the diagnosis of Chronic Obstructive Pulmonary Disease (COPD), as well as predict the severity of the disease. Serum suPAR levels are elevated among COPD patients during exacerbation as compared to stable COPD patients: this provides diagnostic and prognostic advantage to the clinician. Soluble Urokinase Plasminogen Activator Receptor (suPAR) can also be used to assess the response to COPD treatment. It is a specific and independent predictor of mortality among COPD patients with comorbid diseases like congestive heart failure and renal failure.
[1]. Barnes PJ, Shapiro SD, Pauwels RA, Chronic obstructive pulmonary disease: molecular and cellular mechanismsEur Respir J 2003 22(4):672-88.10.1183/09031936.03.0004070314582923 [Google Scholar] [CrossRef] [PubMed]
[2]. Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, BOLD Collaborative Research GroupInternational variation in the prevalence of COPD (the BOLD Study): a population-based prevalence studyLancet 2007 370:741-50.10.1016/S0140-6736(07)61377-4 [Google Scholar] [CrossRef]
[3]. Murray CJ, Lopez AD, Black R, Mathers CD, Shibuya K, Ezzati M, Global burden of disease 2005: call for collaboratorsLancet 2007 370:109-10.10.1016/S0140-6736(07)61064-2 [Google Scholar] [CrossRef]
[4]. Reilly JJ, Silverman EK, Shapiron SD, Chronic Obstructive Pulmonary disease. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editorsHarrison’s principles of internal medicine 2015 19th edition(2)NewyorkThe McGraw-Hill Companies:1700-7. [Google Scholar]
[5]. Hogg JC, Chu F, Utokaparch S, The nature of small airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med 2004 350(26):2645-53.10.1056/NEJMoa03215815215480 [Google Scholar] [CrossRef] [PubMed]
[6]. Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summaryAm J Respir Crit Care Med 2001 163:1256-76.10.1164/ajrccm.163.5.210103911316667 [Google Scholar] [CrossRef] [PubMed]
[7]. Priesto A, Reyes E, Bernstein ED, Martinez B, Monserrat J, Izquierdo JL, Defective natural killer and phagocytic activities in chronic obstructive pulmonary disease are restored by glycophopeptical (Immunoferon)Am J Respir Crit Care Med 2001 163(7):1578-83.10.1164/ajrccm.163.7.200201511401877 [Google Scholar] [CrossRef] [PubMed]
[8]. Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M, Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cellsImmunol Cell Bio 2003 81(4):289-96.10.1046/j.1440-1711.2003.t01-1-01170.x12848850 [Google Scholar] [CrossRef] [PubMed]
[9]. Taylor AE, Hayward TK, Quint JK, Thomas CMR, Tudhope SJ, Wedzicha JA, Defective macrophage phagocytosis of bacteria in COPDEur Respir J 2010 35(5):1039-47.10.1183/09031936.0003670919897561 [Google Scholar] [CrossRef] [PubMed]
[10]. Hodge S, Hodge G, Ahern J, Jersmann H, Holmes M, Reynolds PN, Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol 2007 37:748-55.10.1165/rcmb.2007-0025OC17630319 [Google Scholar] [CrossRef] [PubMed]
[11]. Donadello K, Scolletta S, Covajes C, Vincent JL, suPAR as a prognostic biomarker in sepsisBMC Medicine 2012 10:210.1186/1741-7015-10-222221662 [Google Scholar] [CrossRef] [PubMed]
[12]. Mahmood N, Mihalcioiu C, Rabbani SA, Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic ApplicationsFront. Oncol 2018 8:2410.3389/fonc.2018.0002429484286 [Google Scholar] [CrossRef] [PubMed]
[13]. Gonias SL, Hu J, Urokinase receptor and resistance to targeted anticancer agentsFront. Pharmacol 2015 6:15410.3389/fphar.2015.0015426283964 [Google Scholar] [CrossRef] [PubMed]
[14]. Vassalli JD, Sappino AP, Belin D, The plasminogen activator/plasmin systemJ clin Invest 1991 88(4):1067-72.10.1172/JCI1154051833420 [Google Scholar] [CrossRef] [PubMed]
[15]. Wilhelm OG, Wilhelm S, Escott GM, Lutz V, Magdolen V, Schmitt M, Cellular glycosylphosphatidylinositol specific phospholipase D regulates urokinase receptor shedding and cell surface expressionJ Cell Physiol 1999 180(2):225-35.10.1002/(SICI)1097-4652(199908)180:2<225::AID-JCP10>3.0.CO;2-2 [Google Scholar] [CrossRef]
[16]. Syrovatkina V, Alegre KO, Dey R, Huang XY, Regulation, signaling, and physiological functions of G-proteinsJ Mol Biol 2016 428(19):3850-68.10.1016/j.jmb.2016.08.00227515397 [Google Scholar] [CrossRef] [PubMed]
[17]. Mondino A, Blasi F, uPA and uPAR in fibrinolysis, immunity and pathologyTrends in Immunol 2004 25(8):450-55.10.1016/j.it.2004.06.00415275645 [Google Scholar] [CrossRef] [PubMed]
[18]. Plesner T, Behrendt N, Ploug M, Structure, function and expression on blood and bone marrow cells of the urokinase-type plasminogen activator receptor, uPARStem Cells 1997 15(6):398-408.10.1002/stem.1503989402652 [Google Scholar] [CrossRef] [PubMed]
[19]. Smith HW, Marshall CJ, Regulation of cell signalling by uPARNat Rev Mol Cell Biol 2010 11(1):2310.1038/nrm282120027185 [Google Scholar] [CrossRef] [PubMed]
[20]. Jiang Y, Xiao W, Zhang Y, Xing Y, Urokinase-type plasminogen activator system and human cationic antimicrobial protein 18 in serum and induced sputum of patients with chronic obstructive pulmonary diseaseRespirology 2010 15(6):939-46.10.1111/j.1440-1843.2010.01799.x20624254 [Google Scholar] [CrossRef] [PubMed]
[21]. Bozinovski S, Hutchinson A, Thompson M, MacGregor L, Black J, Giannakis E, Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med 2008 177(3):269-78.10.1164/rccm.200705-678OC18006888 [Google Scholar] [CrossRef] [PubMed]
[22]. Smith DJ, Yerkovich ST, Towers MA, Carroll ML, Thomas R, Upham JW, Reduced soluble receptor for advanced glycation end-products in COPDEur Respir J 2011 37(3):516-22.10.1183/09031936.0002931020595148 [Google Scholar] [CrossRef] [PubMed]
[23]. Zhang Y, Xiao W, Jiang Y, Wang H, Xu X, Ma D, Levels of components of the urokinase-type plasminogen activator system are related to chronic obstructive pulmonary disease parenchymal destruction and airway remodellingJ Int Med Res 2012 40(3):976-85.10.1177/14732300120400031622906270 [Google Scholar] [CrossRef] [PubMed]
[24]. Edsfeldt A, Nitulescu M, Grufman H, Soluble urokinase plasminogen activator receptor is associated with inflammation in the vulnarable human atherosclerotic plaqueStroke 2012 43(12):3305-12.10.1161/STROKEAHA.112.66409423150653 [Google Scholar] [CrossRef] [PubMed]
[25]. Mondino A, Blasi F, uPA and uPAR in fibrinolysis, immunity and pathologyTrends Immunol 2004 25(8):450-55. [Google Scholar]
[26]. Piironen T, Laursen B, Pass J, List K, Gardsvoll H, Ploug M, Hoyer HG, Specific immunoassays for detection of intact and cleaved forms of the urokinase receptorClinical Chemistry 2004 50(11):2059-68.10.1373/clinchem.2004.03823215345662 [Google Scholar] [CrossRef] [PubMed]
[27]. Thuno M, Macho B, Eugen OJ, suPAR: the molecular crystal ballDis markers 2009 27(3-4):157-72.10.1155/2009/50429419893210 [Google Scholar] [CrossRef] [PubMed]
[28]. McClintock D, Zhuo H, Wickersham N, Matthay MA, Ware LB, Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injuryCrit Care 2018 12(2):R4110.1186/cc684618358078 [Google Scholar] [CrossRef] [PubMed]
[29]. Geboers DG, De Beer FM, Boer TDAM, van der Poll T, Horn J, Cremer OL, Plasma suPAR as a prognostic biological marker for ICU mortality in ARDS patientsIntensive Care Med 2015 41(7):1281-90.10.1007/s00134-015-3924-926100127 [Google Scholar] [CrossRef] [PubMed]
[30]. MacSweeney R, McAuley DF, Acute respiratory distress syndromeThe Lancet 2016 388(10058):2416-30.10.1016/S0140-6736(16)00578-X [Google Scholar] [CrossRef]
[31]. Fevang B, Olsen JE, Yndestad A, Brosstad F, Beiske K, Aukrust P, Froland SS, Enhanced levels of urokinase plasminogen activator and its soluble receptor in common variable immunodeficiencyClinical Immunology 2009 131(3):438-46.10.1016/j.clim.2009.01.00719232508 [Google Scholar] [CrossRef] [PubMed]
[32]. Hurst JR, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators: Susceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med 2010 363(12):1128-38.10.1056/NEJMoa090988320843247 [Google Scholar] [CrossRef] [PubMed]
[33]. Kurtipek E, Kesli R, Bekci TT, Eroglu F, Akin B, Kurku H, Assessment of soluble urokinase-type plasminogen activator receptor (suPAR) in chronic obstructive pulmonary diseaseInternational Archives of Medicine 2015 8ISSN 1755-7682. Available at: <https://imed.pub/ojs/index.php/iam/article/view/1057< Date accessed: 04 mar. 2019. doi: http://dx.doi.org/10.3823/162310.3823/1623 [Google Scholar] [CrossRef]
[34]. Solberg H, Ploug M, Hansen G, Nielsen BS, Lund LR, The murine receptor for urokinase-type plasminogen activator is primarily expressed in tissues actively undergoing remodelingJ Histochem Cytochem 2001 49(2):237-46.10.1177/00221554010490021111156692 [Google Scholar] [CrossRef] [PubMed]
[35]. Nykjaer A, Moller B, Todd RF, Christensen T, Andreasen PA, Gliemann J, Urokinase receptor. An activation antigen in human T lymphocytesJ Immunol 1994 152(2):505-16. [Google Scholar]
[36]. Gumus A, Altintas N, Cinarka H, Kirbas A, Haziroglu M, Karatas M, Sahin U, Soluble urokinase-type plasminogen activator receptor is a noval biomarker predicting acute exacerbation in COPDInt J Chron Obstruct Pulmon Dis 2015 10:358-64.10.2147/COPD.S7765425709430 [Google Scholar] [CrossRef] [PubMed]
[37]. AboEl MGH, Mabrouk MM, Soluble urokinase-plasminogen activator receptor as a measure of treatment response in acute exacerbation of COPDJ Bras Pneumol 2018 44(1):36-41.10.1590/s1806-3756201700000015129538541 [Google Scholar] [CrossRef] [PubMed]
[38]. Kurtipek E, Kesli R, Bekci TT, Eroglu F, Akin B, Kurku H, Assessment of soluble urokinase-type plasminogen activator receptor (suPAR) in chronic obstructive pulmonary diseaseInternational Archives of Medicine 2015 8ISSN 1755-7682. Available at: <https://imed.pub/ojs/index.php/iam/article/view/1057> Date accessed: 04 mar. 2019. doi: http://dx.doi.org/10.3823/162310.3823/1623 [Google Scholar] [CrossRef]
[39]. Can U, Guzelant A, Yerlikaya F, Yosunkaya S, The role of serum soluble urokinase-type plasminogen activator receptor in stable chronic obstructive pulmonary diseaseJ Investig Med 2014 62(7):938-43.10.1097/JIM.000000000000010525127435 [Google Scholar] [CrossRef] [PubMed]
[40]. Xiao W, Hsu YP, Ishizaka A, Kirikae T, Moss RB, Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammationChest 2005 128(4):2316-26.10.1378/chest.128.4.231616236890 [Google Scholar] [CrossRef] [PubMed]
[41]. Wang H, Yang T, Li D, Wu Y, Zhang X, Pang C, Elevated circulating PAI-1 levels are related to lung function decline, systemic inflammation, and small airway obstruction in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis 2016 11:236910.2147/COPD.S10740927713627 [Google Scholar] [CrossRef] [PubMed]
[42]. Godtfredsen NS, Jorgensen DV, Marsaa K, Ulrik CS, Andersen O, Eugen-Olsen J, Soluble urokinase plasminogen activator receptor predicts mortality in exacerbated COPDRespiratory Research 2018 19:9710.1186/s12931-018-0803-229783959 [Google Scholar] [CrossRef] [PubMed]
[43]. Bocskei RM, Benczur B, Losonczy G, Illyes M, Cziraki A, Muller V, Soluble urokinase-type plasminogen activator receptor and arterial stiffness in patients with COPDLung 2019 197:189-97.10.1007/s00408-019-00211-w30820636 [Google Scholar] [CrossRef] [PubMed]
[44]. Loukeri A, Spithakis PD, Moschos C, Loukeri P, Bartzeliotou A, Tzagkaraki A, Plasma levels of soluble urokinase plasminogen activator receptor (suPAR) as a possible biomarker for lung cancer and/or COPDEuropean Respiratory Journal 2016 48:PA285110.1183/13993003.congress-2016.PA2851 [Google Scholar] [CrossRef]