Appearance of the Optic Nerve Sheath Diameter (ONSD) using Higher Frequency Linear Probes in Detection and Monitoring of Raised Intracranial Pressures-A Cadaveric Study
Kishore Kumar Pichamuthu1, Ivan James Prithishkumar2
1 Professor, Department of Medical Intensive Care Unit, Christian Medical College, Vellore, Tamil Nadu, India.
2 Professor, Department of Anatomy, Christian Medical College, Vellore, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Ivan James Prithishkumar, Professor, Department of Anatomy, Christian Medical College, Vellore, Tamil Nadu, India.
E-mail: drivanjames@gmail.com
Introduction
Trans-bulbar ultrasonography of Optic Nerve Sheath Diameter (ONSD) is increasingly used to detect raised Intracranial Pressures (ICP). Current guidelines for measuring ONSD are based on earlier descriptions of anatomy of optic nerve sheath using a 7.5 MHz linear probe. There is wide variation in the cut-off value of an abnormal ONSD, mostly due to an edge artefact around the dura and uncertainty over placement of measurement cursors.
Aim
To describe the detailed anatomy of the appearance of ONSD on cadaveric simulated models using a higher frequency probe.
Materials and Methods
A 20G intravenous cannula was inserted into the subarachnoid space around optic nerve of nine embalmed cadavers and the space gradually insufflated with saline to mimic raised ICP. Axial and lateral ultrasonography of ONSD was done using a high frequency 13 MHz transducer, before and after insufflation of the space. The ultrasonic appearance of optic nerve sheath at normal and increased intracranial pressures was studied.
Results
At normal pressures, the true subarachnoid CSF space was either not visible or seen as a thin sliver of anechoic space. At elevated pressures, this space appeared as an anechoic triangular or semi lunar space with scattered trabecular echoes on either side of anterior optic nerve in axial and longitudinal scans. Edge artefacts are easily appreciated.
Conclusion
High frequency ultrasound probes (13 MHz) easily guide identification of duramater, distinguish edge artefacts and observe changes in appearance of subarachnoid space in elevated ICP. This would help in precise bed side monitoring of raised ICP especially in resource poor settings. Images described here can be a useful tool to educate and train ICU and emergency care providers.
Subarachnoid space, Trans-ocular, Ultrasound
Introduction
Identification and monitoring of raised ICP is absolutely essential in several clinical scenarios in the emergency and intensive care units. Raised ICP is often a predictor of poor prognosis. Several non-invasive methods are available to detect raised ICP, the simplest being examination of the fundus with an ophthalmoscope for evidence of papilledema. This often requires an experienced physician. Raised ICP is usually not diagnosed during the early phase, as papilledema appears much later requiring sustained elevations of intracranial pressures. CT (Computer Tomography) and MRI (Magnetic Resonance Imaging) of head can be done to detect raised pressures, but this requires the transfer of a critically ill patient on support systems to the CT/MRI room. CT and MRI may often not be available in resource poor settings [1,2].
The subarachnoid space around the brain extends along the optic nerves to the posterior aspect of the globe where the duramater becomes continuous with the sclera. Few authors have studied the morphometry of optic nerve and its surrounding sheath-the average length of optic nerve is about 40 mm; it’s diameter including the dural sheath is 4 mm; the dural sheath itself is 0.4 mm in diameter; the subarachnoid space around optic nerve has an average width of 0.1 mm and contains about 0.1 mL of cerebrospinal fluid [1,3-5]. In conditions of raised ICP, CSF tends to accumulate in the subarachnoid space around the optic nerve. Sustained pressures cause stretching of the dural sheath and widening of the ONSD. This widening is reported to significantly widen the anterior segment of optic nerve sheath with little or no dilatation of the posterior segment [1,3,6].
Trans-bulbar ultrasonography of the ONSD is now increasingly used to detect raised ICP in the emergency and intensive care units [1,7-13]. Trans-bulbar sonography is quick, non-invasive and easily affordable. However, the current guidelines for measurement of ONSD are based on the sono-anatomy described in studies using a 7.5 MHz linear probe [6,9].
Hence, there is a need to revisit the sono-anatomy of the optic nerve sheath, in normal individuals and those with raised intracranial pressures using currently available, better quality, highly ubiquitous, high frequency probes (>12 MHz). We determined to describe the appearance of the true dura, differentiate it from edge artefacts, delineate the subarachnoid space, and observe the changes in the ultrasonic appearance of subarachnoid space and ONSD in cadaver orbital preparations with simulated raised subarachnoid pressure.
Materials and Methods
This experimental study was done over a period of six months from Jan 2016-June 2016 and permission obtained from the institutional review board (IRB no. 10454). The study design is a pre-post subtype of experimental (interventional) study, where comparison is made before and after an intervention, in this case artificially simulated raised intracranial pressures.
The cadaveric study was done in the dissection lab of Department of Anatomy, Christian Medical College, Vellore. Inclusion criteria were: cadavers with intact eye globes and periorbital soft tissue content, with intact meningeal sheathes around the optic nerve. Exclusion criteria were: those cadavers in which the eye globe was removed for eye donation purposes or those with damaged meningeal sheaths around the optic nerve.
Nine embalmed cadavers donated to the department of anatomy under the body donation program were chosen. After removing the scalp, the vault of skull was opened using a fine hand saw 1 cm above the superciliary arch in front and the external occipital protuberance posteriorly, and the brain was carefully removed. The optic nerve was bisected at its junction with the chiasma. The bony roof of orbit and optic canal was carefully removed to expose the orbital contents and optic nerve. The posterior end of the optic nerve was released from the bony optic canal. The subarachnoid space around the cut end of optic nerve was identified and a 20G intravenous cannula was inserted into the subarachnoid space. The proximal end of the optic nerve together with the cannula was tightly ligated. The cannula was connected to a stopcock and a syringe (5 cc) filled with normal saline (0.9%).
The entire head and neck section was immersed in water for the ultrasound study to prevent interference from air pockets in the orbital tissues. Scans were performed under water using a 13 MHz linear transducer (Sonosite Micromaxx®). Axial and lateral ultrasound measurements were taken before and after insufflating the space with fluid under increasing pressure. Axial scans were performed with the transducer on the eyelids directed posteriorly. Longitudinal scans were performed with the transducer placed longitudinally on the superior aspect of the optic nerve and the adjacent part of the globe. In both views, the subarachnoid space was insufflated by injecting it gradually with saline from a syringe. Measurements were made with electronic calipers on images before and after insufflation at a fixed landmark (0.30 cm or 3 mm) behind the optic nerve head or papilla. Images were captured using the ultrasound and colour modifications were made to denote the subarachnoid space before and after insufflation. Subsequently, scans were repeated after removal of dural layer on one side of the optic nerve. As this study dealt with the ultrasonic appearance of the perineural subarachnoid space, the widening of ONSD and changes in its appearance pre- and post-insufflation are accurately described.
Results
We present several descriptive images using axial, lateral and longitudinal scans using a 13 MHz probe. The pia and dura-arachnoid may appear as one layer at normal CSF pressures. The true dura could be identified by tracing its curvature away from the optic nerve head and merging with the scleral layers, while the pia-arachnoid arcs inwards towards the optic nerve head [Table/Fig-1,2].
The true dura-arachnoid can be identified by tracing its curvature away from the optic nerve head where it merges with the sclera, while the pia arcs inwards towards the optic nerve head.
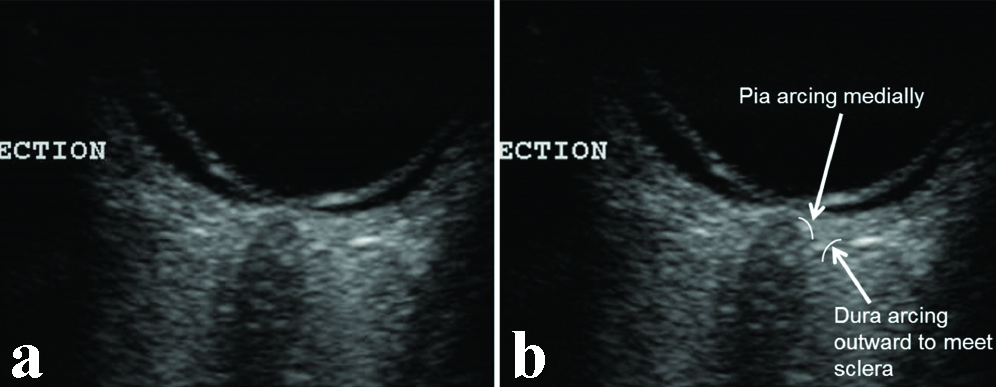
The image traces the dura and pia forwards. The edge artefact can also be seen. A minimal amount of CSF in the subarachnoid space is seen near the optic nerve head.
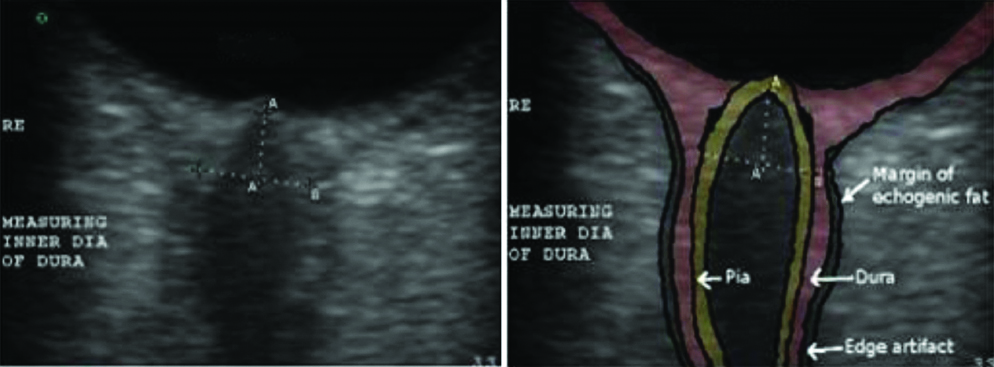
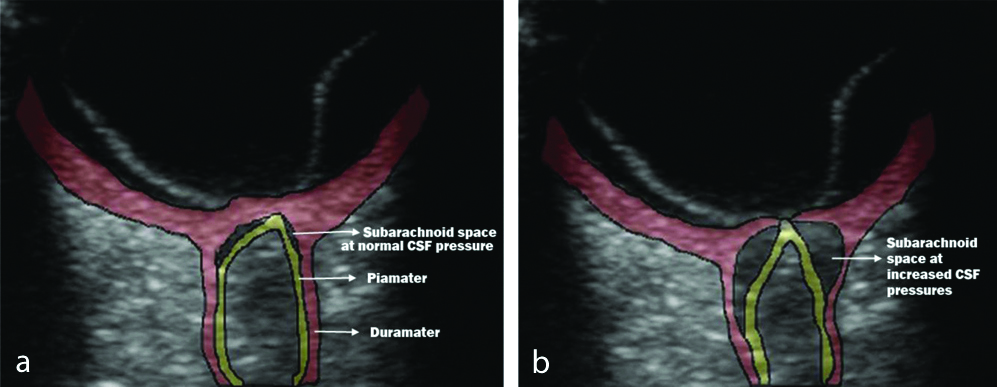
At normal pressures, the true subarachnoid CSF space was either not visible or seen as a thin sliver of anechoic space [Table/Fig-3]. At elevated CSF pressures this space was clearly seen as an anechoic triangular or semi lunar space with scattered trabecular echoes on either side of the anterior optic nerve [Table/Fig-3]. The widening of the ONSD is also clearly seen in [Table/Fig-3,4]. The distensibility of this space was maximal 3 mm behind the papilla. The subarachnoid space tapered posteriorly 6-7 mm behind the papilla. This is in contrast to edge artefacts which maintain their thickness or widen posteriorly. A thin anechoic edge artefact could be seen separating the dura from the echogenic fat, particularly at normal CSF pressures [Table/Fig-2].
a) At normal pressures, the true subarachnoid CSF space; b) A thin anechoic edge artefact can be seen separating the dura from the echogenic fat; at elevated CSF pressures the subarachnoid space.
Longitudinal scans show the subarachnoid space and ONSD in the pre- and post-insufflated state. ‘A’ represents the fixed landmark (0.30 cm or 3 mm) behind the optic nerve head or papilla where measurements of ONSD was taken. ‘B’ represents the marked widening of the ONSD from 0.47 cm in the pre-insufflated state to 0.61 cm post insufflation with saline to mimic raised intracranial pressures.
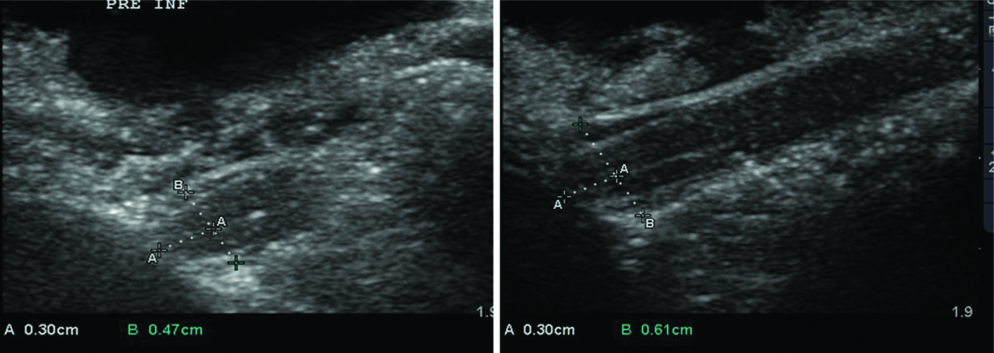
Longitudinal scans were performed with the transducer placed longitudinally on the superior aspect of optic nerve and adjacent part of the globe. The widening of the subarachnoid space and ONSD can be clearly appreciated while insufflating with saline [Table/Fig-4].
Discussion
Identification and monitoring of raised ICP is crucial, urgent and lifesaving in several medical emergencies. Examination of the fundus using an ophthalmoscope is perhaps the simplest way of diagnosing raised pressures. CT and MRI of head can also be done but is inconvenient, requiring the transfer of a critically ill patient on support systems [1,2]. Trans-bulbar ultrasonography of the ONSD can be done at the bedside, is quick, non-invasive and easily affordable. However, the ultrasonic appearance of optic nerve, its sheath and the subarachnoid space using high frequency probes (>12 MHz) has not been described adequately. The best descriptions are those by Hansen and Helmke in 1996 using a 7.5 MHz probe, and the current guidelines for measurement of ONSD are based on them [6].
Though ONSD has been well evaluated, there is no consensus on the cut-off value of an abnormal ONSD indicating raised ICP. There is considerable inter-individual variation in the threshold measurement of ONSD in literature [Table/Fig-5] [7,14-19].
Shows the wide variation in the measurement of ONSD in patients with raised ICP and their controls [7,14-19].
| Author, Year | No. of patients | Ultrasound frequency (Mhz) | Patient (Pts)/Control group | Mean ONSD (mm) | Optimal cut off (mm) [Sensitivity: specificity] |
|---|
| Blaivas M et al., 2003 [7] | 35 | 10 | Pts without raised ICP | 4.4 | 5.0 mm(Sens-100%: spec-95%) |
| Pts with raised ICP | 6.2 |
| Geerearts T et al., 2007 [14] | 31 | 7.5 | Pts without raised ICP | 5.1 | 5.9 mm(Sens-87%: spec-94%) |
| Pts with raised ICP | 6.2 |
| Controls | 4.9 |
| Kimberly HH et al., 2008 [15] | 15 | 10 | Pts without raised ICP | 4.4 | 5.0 mm |
| Pts with raised ICP | 5.4 |
| Soldatos T et al., 2008 [16] | 67 | 9 | Pts with raised ICP | 6.1 | 5.7 mm(Sens-74%: spec-100%) |
| Controls | 3.6 |
| Geeraerts T et al., 2008 [17] | 37 | 7.5 | Pts with/without raised ICP | - | 5.86 mm(Sens-90%: spec-84%) |
| Venkatakrishna R et al., 2011 [18] | 65 | 13 | Pts without raised ICP | 4.0 | 4.8 mm(Sens-96%: spec-94%) |
| Pts with raised ICP | 5.3 |
| Amini A et al., 2013 [19] | 50 | 7.5 | Pts without raised ICP | 4.6 | 5.5(Sens-100%: spec-100%) |
| Pts with raised ICP | 6.7 |
[Table/Fig-5] shows the wide variation of ONSD in patients with raised ICP and their controls. Most of these studies have used ultrasonic probes with 7-10 MHz frequencies. [Table/Fig-6] shows the wide variation of ONSD in normal healthy adults [20-23]. Three possible causes of wide variations in ONSD cutoff include: a) Use of lower frequency probes-Technology has rapidly progressed over time. Most previous studies use the technique described by Hansen and Helmke in 1996 where a 7.5 MHz probe was used and cursors placed at the hyperechoic margins formed by the periorbital fat on either side of the hypoechoic band consisting of the nerve and the perineural space 3 mm behind the globe [4,6]. However, currently available machines use high frequency transducers (>12 MHz) and have better quality of images. Images acquired now are very different from those described 18 years ago; b) Inclusion of an edge artefact around the true dura causing a wider ONSD. Lower frequency probes do not clearly differentiate the margins of optic nerve sheath; and c) Thirdly, there is uncertainty over placement of measurement cursors. The term “3 mm behind the globe” has been interpreted by some to be 3 mm behind the posterior margin of the sclera instead of 3 mm behind the optic nerve head or papilla [24].
Shows the wide variation in ONSD values (range and mean) in normal healthy living adults [20-23].
| Author, year | No. patients | Ultrasound frequency (MHz) | ONSD range in healthy subjects (MM) | ONSD mean (MM) |
|---|
| Ballantyne SA et al., 2002 [20] | 67 | 7 | 2.4-4.7 | 3.3 |
| Garcia JPS et al., 2004 [21] | 32 | 10 | 3.9-5.9 | 4.8 |
| Potgieter DW et al., 2011 [22] | 12 | 7.5 | 1.3-5.5 | - |
| Bauerle J et al., 2012 [23] | 40 | 9 | 4.3-7.6 | 5.4 |
No previous studies have described in detail the appearance of ONSD and the subarachnoid space using high frequency probes (>12 MHz). Ours is the first reported study. The higher quality images offer easy identification of meningeal layers thus enabling correct placement of measurement cursors to measure and monitor ONSD. These descriptions would certainly help in easier but more precise bed side monitoring of patients with raised ICP, especially in resource poor settings. The several high-quality images at various axes described in this study can also be a useful tool to educate and train emergency care providers and ultrasonologists.
Limitation
The limitation of our study is perhaps the smaller sample size as most cadavers donated to us have their eyeballs removed for the eye donation program. Also, we mimicked raised intracranial pressures by inflating the subarachnoid space with normal saline.
Conclusion
In conclusion, the sono-anatomy of optic nerve sheath described by us using high frequency ultrasound probes (13 MHz) can easily guide accurate identification of the duramater, clearly differentiate artefacts and observe changes in the appearance of the subarachnoid space in elevated intracranial pressures. It is our sincere hope, that more studies are done to describe the detailed ultrasonic appearance of ONSD using higher frequency probes and that bedside trans-ocular ultrasonography would be more frequently used to detect raised intracranial pressures.
[1]. Soldatos T, Chatzimichail K, Optic nerve sonography: A new window for the non-invasive evaluation of intracranial pressure in brain injuryEmerg Med J 2009 26(9):630-34.10.1136/emj.2008.05845319700575 [Google Scholar] [CrossRef] [PubMed]
[2]. Maude RR, Hossain MA, Hassan MU, Osbourne S, Sayeed KL, Karim MR, Transorbital sonographic evaluation of normal optic nerve sheath diameter in healthy volunteers in BangladeshPLoS ONE 2013 8(12):e8101310.1371/journal.pone.008101324312515 [Google Scholar] [CrossRef] [PubMed]
[3]. Liu D, Kahn M, Measurement and relationship of subarachnoid pressure of the optic nerve to intracranial pressures in fresh cadaversAm J Ophthalmol 1993 116:548-56.10.1016/S0002-9394(14)73195-2 [Google Scholar] [CrossRef]
[4]. Barr R, Gean A, Craniofacial trauma. In: Brant W, Helms C (eds)Fundamentals of diagnostic radiology 1999 2nd edPhiladelphiaLippincott Williams & Wilkins:49-61. [Google Scholar]
[5]. Killer HE, Laeng HR, Flammer J, Groscurth P, Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerationsBr J Ophthalmol 2003 87(6):777-81.10.1136/bjo.87.6.77712770980 [Google Scholar] [CrossRef] [PubMed]
[6]. Hansen HC, Helmke K, The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheathSurg Radiol Anat 1996 18:323-28.10.1007/BF01627611 [Google Scholar] [CrossRef]
[7]. Blaivas M, Theodoro D, Sierzenski PR, Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheathAcademic Emergency Medicine 2003 10:376-81.10.1197/aemj.10.4.37612670853 [Google Scholar] [CrossRef] [PubMed]
[8]. Kim DH, Jun JS, Kim R, Ultrasonographic measurement of the optic nerve sheath diameter and its association with eyeball transverse diameter in 585 healthy volunteersSci Rep 2017 7(1):1590610.1038/s41598-017-16173-z29162911 [Google Scholar] [CrossRef] [PubMed]
[9]. Sahu S, Swain A, Optic nerve sheath diameter: A novel way to monitor the brainJ Neuroanaesthesiol Crit Care 2017 4(Suppl S1):13-18.10.4103/jnacc-jnacc-73.16 [Google Scholar] [CrossRef]
[10]. Williams P, Optic nerve sheath diameter as a bedside assessment for elevated intracranial pressureCase Rep Crit Care 2017 2017:397893410.1155/2017/397893410.1155/2017/397893428480083 [Google Scholar] [CrossRef] [CrossRef] [PubMed]
[11]. Weinmann AV, Beaucreux C, Kearns K, Dubost C, Keep an eye on the intracranial pressure, thanks to the optic nerve sheath diameterIndian J Crit Care Med 2018 22(6):460-62.10.4103/ijccm.IJCCM_446_1729962750 [Google Scholar] [CrossRef] [PubMed]
[12]. Yanamandra U, Gupta A, Bhattachar SA, Yanamandra S, Das SK, Patyal S, Comparison of optic nerve sheath diameter between both eyes: a bedside ultrasonography approachIndian J Crit Care Med 2018 22(3):150-53.10.4103/ijccm.IJCCM_498_1729657371 [Google Scholar] [CrossRef] [PubMed]
[13]. Wang LJ, Chen LM, Chen Y, Bao LY, Zheng NN, Wang YZ, Ultrasonography assessments of optic nerve sheath diameter as a noninvasive and dynamic method of detecting changes in intracranial pressureJAMA Ophthalmol 2018 136(3):250-56.10.1001/jamaophthalmol.2017.656029392301 [Google Scholar] [CrossRef] [PubMed]
[14]. Geeraerts T, Launey Y, Martin L, Pottecher J, Vigué B, Duranteau J, Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injuryIntensive Care Med 2007 33:1704-11.10.1007/s00134-007-0797-617668184 [Google Scholar] [CrossRef] [PubMed]
[15]. Kimberly HH, Shah S, Marill K, Noble V, Correlation of optic nerve sheath diameter with direct measurement of intracranial pressureAcad Emerg Med 2008 15(2):201-04.10.1111/j.1553-2712.2007.00031.x18275454 [Google Scholar] [CrossRef] [PubMed]
[16]. Soldatos T, Karakitsos D, Chatzimichail K, Papathanasiou M, Gouliamos A, Karabinis A, Optic nerve sonography in the diagnostic evaluation of adult brain injuryCrit Care 2008 12:1-7.10.1186/cc689718477382 [Google Scholar] [CrossRef] [PubMed]
[17]. Geeraerts T, Merceron S, Benhamou D, Vigué B, Duranteau J, Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patientsIntensive Care Med 2008 34(11):2062-67.10.1007/s00134-008-1149-x18509619 [Google Scholar] [CrossRef] [PubMed]
[18]. Venkatakrishna R, Monique V, Jeffrey JF, Teresa LJ, Optic nerve ultrasound for the detection of raised intracranial pressureNeurocritical Care 2011 15(3):506-15.10.1007/s12028-011-9606-821769456 [Google Scholar] [CrossRef] [PubMed]
[19]. Amini A, Kariman H, Arhami DA, Hatamabadi HR, Derakhshanfar H, Mansouri B, Use of the sonographic diameter of optic nerve sheath to estimate intracranial pressureAmerican Journal of Emergency Medicine 2013 31:236-39.10.1016/j.ajem.2012.06.02522944553 [Google Scholar] [CrossRef] [PubMed]
[20]. Ballantyne SA, O’Neill G, Hamilton R, Hollman AS, Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adultsEur J Ultrasound 2002 15:145-49.10.1016/S0929-8266(02)00036-8 [Google Scholar] [CrossRef]
[21]. Garcia Jr JPS, Garcia PT, Rosen RB, Finger PT, A 3-dimensional ultrasound C-scan imaging technique for optic nerve measurementsOphthalmology 2004 111(6):1238-43.10.1016/j.ophtha.2003.10.02615177978 [Google Scholar] [CrossRef] [PubMed]
[22]. Potgieter DW, Kippin A, Ngu F, McKean C, Can accurate ultrasonographic measurement of the optic nerve sheath diameter (a non-invasive measure of intracranial pressure) be taught to novice operators in a single training session?Anaesth Intensive Care 2011 39:95-100.10.1177/0310057X110390011621375098 [Google Scholar] [CrossRef] [PubMed]
[23]. Bäuerle J, Lochner P, Kaps M, Nedelmann M, Intra- and interobsever reliability of sonographic assessment of the optic nerve sheath diameter in healthy adultsJ Neuroimaging 2012 22:42-45.10.1111/j.1552-6569.2010.00546.x21121998 [Google Scholar] [CrossRef] [PubMed]
[24]. Beare NAV, Kampondeni S, Glover SJ, Molyneux E, Taylor TE, Harding SP, Detection of raised intracranial pressure by ultrasound measurement of optic nerve sheath diameter in African childrenTrop Med Int Health 2008 13:1400-04.10.1111/j.1365-3156.2008.02153.x18983275 [Google Scholar] [CrossRef] [PubMed]