Syringocystadenocarcinoma Papilliferum (SCACP) is a rare adnexal malignant neoplasm. Most of the cases present with a long standing mass with a sudden progression in size. A 60-year-old female presented with history of swelling over the antero-lateral aspect of left thigh since three years. The lesion was associated with history of pain and ulceration. Grossly, the external surface showed three ulcerated areas. The largest ulcer measured 4×5.5 cm. On cut section, the tumour showed variegated appearance consisting of grey-white to pink areas, multiple cysts, focal necrosis and hemorrhagic areas. Microscopically, tumour cells were arranged in papillary configuration, solid sheets, tubular and acinar pattern. Individual tumour cells showed pleomorphic vesicular nucleus and mitotic figures. The intervening connective tissue showed dense chronic inflammatory cell infiltrate composed of lymphocytes, plasma cells and eosinophils. Also seen were areas of necrosis and Gamna-Gandy body. Based on histological features, a diagnosis of malignant appendageal tumour of the skin with apocrine differentiation, favouring SCACP was offered. By Immunohistochemistry (IHC), tumour cells showed focal positivity for CEA and negative for GCDFP-15. IHC may be helpful, but a pathologist has to primarily depend on the histopathological characteristics of the lesion for diagnosing the condition.
Case Report
A 60-year-old female presented with history of swelling over the antero-lateral aspect of the left thigh since three years. The patient also gave a history of rapid progression in the size of the swelling since six months. It was associated with history of pain and ulceration since three months. It was associated with fever of four days duration. Past history, family history and personal history were not significant. On local examination, antero-lateral aspect of left thigh showed a swelling measuring about 8 cm in greatest dimension. The swelling was firm in consistency, tender and showed restricted mobility and showed multiple ulcerated areas. The largest ulcer measured 5.5 cm in greatest dimension. The ulcer showed serous discharge, irregular margins, rolled out edges, indurated base and the floor was covered with slough. The surrounding skin was stretched and shiny.
Haematological investigation revealed leucocytosis (total leukocyte count-11,400/mm3), mild thrombocytosis (4.08 lacs/mm3) and eosinophilia (934 cells/mm3). ESR was elevated (70 mm/1st hour). Other investigations like plasma glucose, serum electrolytes and renal function tests were within normal limits. The microbiological investigation (culture) showed no growth. Based on the presentation of the lesion, clinically, a working diagnosis of fibrosarcoma was considered. FNAC was not performed since it was an ulcerated lesion. Incisional biopsy was performed. The biopsy was superficial. The skin tissue displayed ulcerated epidermis and scanty tumor tissue composed of epithelial cells arranged in tubules and cords. Also seen were chronic inflammatory cell infiltrate, chondroid and myxoid areas. Possibility of an adnexal skin neoplasm (chondroid syringoma) was suspected. Since the biopsy was superficial, excision of the tumour was suggested. Wide local excision of the tumour was performed along with dissection of inguinal and external iliac lymph nodes. The specimen was sent for histopathological examination.
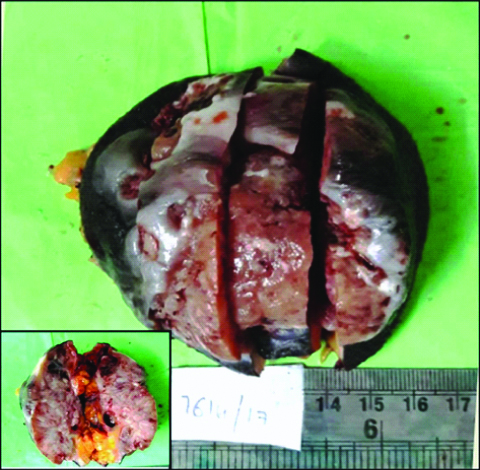
Grossly, the skin covered soft tissue mass measured 9×8×4.5 cm. The overlying skin measured 8×9 cm. External surface showed three ulcerated areas and the largest ulcer measured 4×5.5 cm. On cut section, the tumour measured 7×7×3.5 cm and showed variegated appearance consisting of grey-white to pink areas, multiple cysts, focal necrosis and hemorrhagic areas. Largest cystic space measured 2.5×1.8 cm [Table/Fig-1]. Six lymph nodes (single external iliac lymph node and five inguinal lymph nodes) were isolated. Cut section of the lymph nodes showed grey brown areas.
Gross photograph of skin covered tumor tissue displaying multiple ulcers. Inset: Cut section show grey-pink fleshy tumor with variegated areas composed of multiple cysts, focal necrosis and hemorrhagic areas.
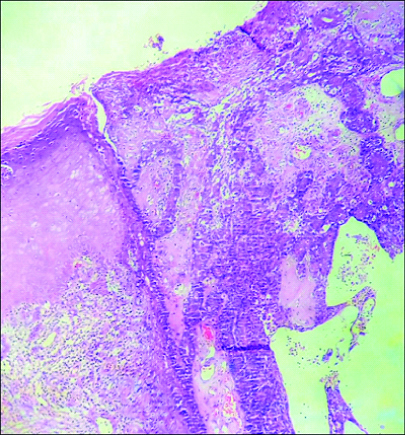
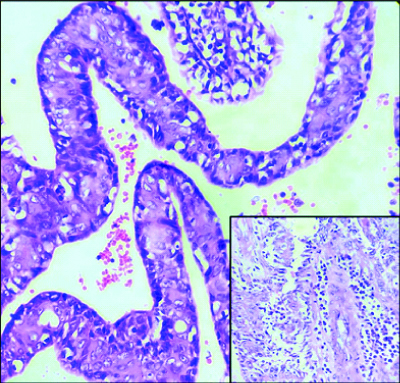
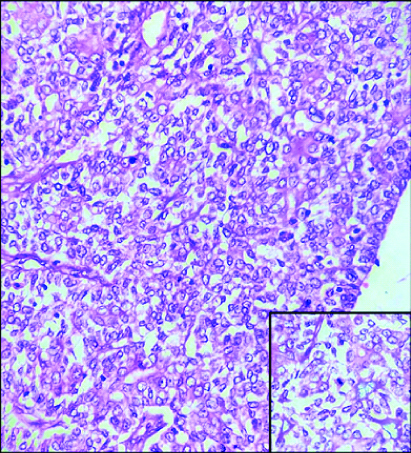
Multiple histological sections studied showed epidermis, dermis and subcutaneous tissue. Epidermis showed focal ulceration, focal hyperkeratosis, parakeratosis and irregular acanthosis. The dermis showed a cystic lesion communicating with stratified squamous surface epithelium [Table/Fig-2]. The cystic invagination was lined by inner columnar and outer cuboidal epithelium [Table/Fig-3]. Also seen were tumour cells arranged in papillary configuration with central fibrovascular core and peripheral tumour cells. At places tumour cells were arranged in solid sheets, tubular and acinar pattern. Individual tumour cells were medium sized, round to oval to elongated cells with moderate amount of cytoplasm and pleomorphic vesicular nucleus. Some of the tumour cells showed noticeable nucleoli and mitotic figures [Table/Fig-4]. The intervening connective tissue stroma showed dense chronic inflammatory cell infiltrate composed of lymphocytes, plasma cells and eosinophils. At places luminal cells showed decapitation of secretion into the lumen. There were areas showing haemorrhage, sclerosis, comedo like necrosis, microabscessess, myxoid areas, giant cell reaction and Gamna-Gandy body [Table/Fig-5]. The tumour tissue was seen to infiltrate into surrounding connective tissue at places. All the margins were free from tumour. Sections studied from lymph nodes showed no metastasis.
Microphotograph of tissue section showing epidermis displaying irregular acanthosis and focal ulceration. Cystic tumour tissue is seen to communicate with surface epithelium (H&E, 100X).
Microphotograph of tissue section showing papillary fronds lined by inner columnar and outer cuboidal cells. (H&E, 400X). Inset: Lymphoplasmacytic infiltration (H&E, 400X).
Microphotograph of tissue section showing tumor cells arranged in solid sheets. (H&E, 400X). Inset: Tumor cells show noticeable nucleoli and mitotic figure (H&E, 400X).
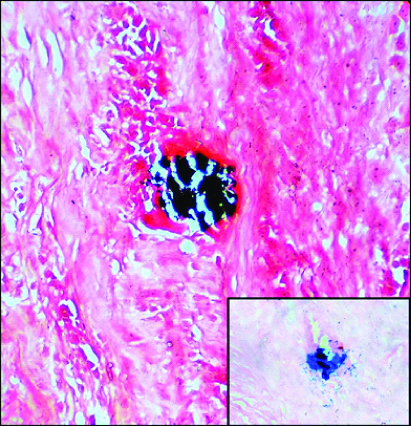
Microphotograph of tissue section showing Gamna-Gandy body (VonKossa, 400X). Inset: Gamna-Gandy body (Perls stain, 400X).
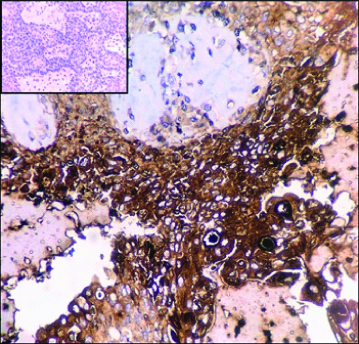
Based on the histological features, a diagnosis of malignant appendageal tumour of the skin with apocrine differentiation, favouring SCACP was suggested. Possibility of metastatic adenocarcinoma as differential diagnosis was ruled out based on characteristic histomorphology and clinical details. Immunohistochemistry (IHC) was performed. The tumour cells showed focal positivity for Carcinoembryonic Antigen (CEA) and were negative for Gross cystic fluid disease protein-15 (GCDFP-15) [Table/Fig-6]. IHC supported the morphological diagnosis of SCACP. The postoperative period was uneventful and the patient was stable at the time of discharge.
Microphotograph of tissue section showing neoplastic cells displaying focal positivity for CEA. (IHC, 400X). Inset: Neoplastic cells negative for GCDFP-15 (IHC, 400X).
Discussion
Adnexal tumours are skin tumours showing differentiation towards one or more of the cutaneous adnexal structures (apocrine, follicular and/or sebaceous) [1]. SCACP is considered by WHO as the malignant counterpart of the more common benign neoplasm [2,3]. Syringocystadenoma papilliferum (SCAP) was described by Stokes in 1917 [4-6]. Dissanayake and Salm were the first to report cases of SCACP in 1980 [3]. SCACP is a rare malignant adnexal neoplasm predominantly showing apocrine differentiation [1]. It has a predilection for head and neck region in the middle aged or elderly individuals [7]. Only few cases have been reported in the literature and its clinical-pathological characteristics are not well defined [8].
The lesion, most commonly affects the elderly and middle aged individuals with a male to female ratio of 1:1 [2,7]. Leeborg N et al., described a case of SCACP in a female in her ninth decade [7]. Park SH et al., and Abrari A et al., documented the lesion in male patients in their seventh decade in their case reports [2,9]. In the present case, the lesion was seen in a female patient in her seventh decade of life.
SCACP is often found in the head and neck region. But it can also occur in the back, chest, arm suprapubic region, perianal region and calf [7,10]. Rao SVS et al., reported a case of SCACP occurring on the right upper arm [3]. Paradiso B et al., described a case in which lesion was located in left shoulder [5]. Leeborg N et al., reported a case in which the patient presented with left neck mass [7]. Abrari A et al., recorded a case in which the lesion was located in axilla [9]. Park SH et al., documented a case in which the patient presented with supra-pubic lesion [2]. In the present case, the lesion was located in the antero-lateral aspect of the left thigh, which is an unusual location for the lesion.
Clinically, these lesions present as skin colored or yellowish papules or nodules that remain unchanged for many years. But it may be associated with sudden progression in the size of the lesion [2]. It may be also associated with painful ulceration that often has secretion oozing out [10]. Similar to the present case, Park SH et al., had documented a case in which the patient had swelling for two years that increased in size and was associated pain and haemorrhage [2].
In the present case, clinically, fibrosarcoma was considered as working diagnosis, based on the presentation of the lesion. Clinically, differential diagnoses include squamous cell carcinoma, basal cell carcinoma and malignant melanoma. However, histological differences between these tumours are well defined [6].
Park SH et al., Leeborg N et al., and Peterson J et al., observed histopathological features similar to that which is documented in the present case report [2,7,10]. These tumours appear to have an overall histologic configuration similar to SCAP but the malignant component may be recognized by nuclear atypia, multilayering, increased mitotic activity including abnormal forms and dermal involvement in those cases associated with an invasive component [11,12]. In the present case, Gamna-Gandy body was observed within the lesion. Gamna-Gandy body represents organized foci of haemorrhage. It is a tell-tale sign of vascular injury within the lesion.
Histopathologically, primary or metastatic adenocarcinoma of other malignant tumours may be considered as differential diagnoses. Some of the malignant tumours of gastrointestinal tract, breast, thyroid, renal cell carcinoma and other tumours may metastasize to the skin at an advanced stages [6]. However, SCACP show characteristic histomorphological features. In the present case, the lesion was thought to be chondroid syringoma on incisional biopsy This was because the biopsy was superficial and non-representative of the lesion. However, excision biopsy was suggested which reveled the actual pathology.
Paradiso B et al., suggested SCACP could be derived from pluripotent stem cells and have Epithelial Mesenchymal Transition (EMT)-like properties. Positivity of the tumour cells for nestin, CD44 and CD133 antigens suggested origin of the tumour from pluripotent stem cells. Strong immunopositivity for CK7, p63 and c-kit suggested EMT-like properties of the tumour [5]. But, the author had performed immunohistochemistry panel on only a single case.
The immunohistochemical expression profile of these tumours shows a mixture of both luminal and basal cell types with variable distribution [13]. Immunoprofile of apocrine tumours show positivity for CEA, BRST-1, GCDFP-15 and CK15 [13,14]. In the present case, neoplastic cells were focally positive for CEA and negative for GCDFP-15. Park SH et al., documented imunoreactivity to only GCDFP-15 [2]. Leeborg N et al., found that the tumour cells were positive for only CEA and Ishida-Yamamoto A et al., observed immunoreactivity to only Human Milk Fat Globule (HMFG-1) [7,8]. Ishida-Yamamoto A et al., stated that loss of immunoreactivity to GCDFP-15 and CEA in SCACP might be associated with dedifferentiation [8]. But, other studies opined that loss of immunoreactivity to GCDFP-15 and CEA were not associated with dedifferentiation [2]. Leeborg N et al., opined that there is no definitive immunoprofile that can discriminate between primary cutaneous and a metastatic adenocarcinoma [7]. Immunophenotypic characteristics of the tumour appear to be variable and inconsistent. Hence we may have to give importance to morphology of the tumour.
Most lesions seem to have arisen from long-standing SCAP [2]. The benign precursor usually antedates the malignancy by many years as a non-progressive verrucous lesion [9]. Also, there is a frequent association with nevus sebaceous and linear nevus verrucosus [10]. Muthusamy RK et al., documented a case of SCACP with coexisting trichoblastoma [4]. [Table/Fig-7] depicts the summary of few cases of SCACP that has been reported in the past [1-11,15-24]. Although rare, this neoplasm needs to be recognized by the surgical pathologists, as the patients diagnosed as SCACP so far, have done well with surgical excision alone [7].
Reported cases of SCACP [1-11,15-24].
| Authors | Age [yrs] | Gender | Location | Clinical presentation | Salient features |
|---|
| Dissanyake and salm [15] | 74 | Female | Scalp | Recent increase in size of the lesion | In-situ carcinoma |
| Dissanayake and Salm [15] | 71 | Female | Back | Present since many years | No recurrence after seven years |
| Seco Navedo MA et al., [16] | 50 | Female | Scalp | Recent increase in size of the lesion | Regional lymph node metastasis |
| Numata M et al., [17] | 52 | Female | Chest | Painful enlarging lesion | Regional lymph node metastasis |
| Bondi and Urso [18] | 47 | Male | Scalp | Present since many years | - |
| Ishida-Yamamoto A et al., [8] | 61 | Male | Perianal | Painful exophytic lesion | In-situ carcinoma with pagetoid spread |
| Arai Y et al., [19] | 64 | Male | Scalp | Associated with bleeding | In-situ carcinoma |
| Chi CC et al., [20] | 60 | Male | Auricle | Painful ulcerated prutitic lesion | Treated with Moh micrographic surgery |
| Wostenborghs H et al., [21] | 81 | Female | Scalp | Associated with bleeding | In-situ carcinoma with pagetoid spread |
| Park SH et al., [2] | 65 | Male | Suprapubic | Painful lesion | No recurrence after two years |
| Langner and Ott [22] | 83 | Male | Perianal | Nodular lesion | In-situ carcinoma |
| Sroa N et al., [23] | 77 | Male | Calf | Present since nine years | Unusual location |
| Leeborg N et al., [7] | 86 | Female | Neck | Exophytic lesion | In-situ carcinoma and squamous differentiation |
| Hoekzema R et al., [24] | 83 | Female | Arm | Present since seven years | Unusual location |
| Ayadin OE et al., [6] | 67 | Male | Scalp | Ulcerated lesion | No recurrence after two years |
| Abrari A et al., [9] | 62 | Male | Axilla | Excoriated lesion | In-situ carcinoma |
| Choccalingam C et al., [1] | 46 | Female | Scalp | Ulcerated lesion | Wide local excision with split-skin graft |
| Peterson J et al., [10] | 65 | Male | Scalp | Exophytic lesion | Arising from SCAP |
| Paradiso B et al., [5] | 88 | Male | Shoulder | Painful erythematous lesion | Squamous differentiation |
| Rao SVS et al., [3] | 53 | Female | Arm | Recent increase in size of the lesion | Unusual location |
| Iga N et al., [11] | 76 | Male | Perianal | Ulcerated lesion | Pagetoid spread |
| Muthuswamy RK et al., [4] | 78 | Female | Scalp | Painful ulcerated lesion | Coexsiting trichoblastoma |
| Present case (2019) | 60 | Female | Thigh | Painful ulcerated lesion | Unusual location and Gamna-Gandy body |
Limitation
FNAC could not be performed since it was an ulcerated lesion. Follow-up of the patient could not be done. Only limited IHC markers were used. IHC has limited role in confirmation of the lesion. IHC may be helpful, but primarily, the diagnosis depends on the histopathological characteristics of the lesion.
Conclusion
SCACP is an entity which poses diagnostic challenge due its rarity and unusual presentation. The present case had unusual location and was histologically associated with Gamna-Gandy body. SCACP is such a lesion in which IHC may be helpful, but a pathologist has to primarily depend on the histopathological characteristics of the lesion for diagnosing the condition.
[1]. Choccalingam C, Samuel P, Subramaniam D, Hammed F, Purushothaman V, Joshi R, Syringocystadenocarcinoma papilliferum: A case report of a rare skin adnexal tumourOur Dermatol Online 2013 4:221-23.10.7241/ourd.20132.54 [Google Scholar] [CrossRef]
[2]. Park SH, Shin YM, Shin SH, Choi JS, Kim KH, Syringocystadenocarcinoma papilliferum: A case reportJ Korean Med Sci 2007 22:762-65.10.3346/jkms.2007.22.4.76217728526 [Google Scholar] [CrossRef] [PubMed]
[3]. Rao SVS, Thirumalasetti N, Syringocystadenocarcinoma papilliferum of arm: A rare case reportNational Journal of Laboratory Medicine 2014 3:10-12. [Google Scholar]
[4]. Muthusamy RK, Mehta SS, Syringocystadenocarcinoma papilliferum with coexisting trichoblastoma: A case report with review of literatureIndian J Dermatol Venereol Leprol 2017 83:574-76.10.4103/ijdvl.IJDVL_755_1628656914 [Google Scholar] [CrossRef] [PubMed]
[5]. Paradiso B, Bianchini E, Cifelli P, Cavazzini L, Lanza G, A new case of syringocystadenocarcinoma papilliferum: A rare pathology for a wide-ranging comprehensionCase Reports in Medicine 2014 2014:453874http//dx.doi/10.1155/2014/45387410.1155/2014/45387424959179 [Google Scholar] [CrossRef] [PubMed]
[6]. Ayadin OE, Sahin B, Ozkan HS, Gore O, A rare tumor: Syringocystadenocarcinoma papilliferumDermatol Surg 2011 37:271-74.10.1111/j.1524-4725.2011.01872.x21324034 [Google Scholar] [CrossRef] [PubMed]
[7]. Leeborg N, Thompson M, Rossmiller S, Gross N, White C, Gatter K, Diagnostic pitfalls in syringocystadenocarcinoma papilliferum: Case report and review of the literatureArch Pathol Lab Med 2010 134:1205-09. [Google Scholar]
[8]. Ishida-Yamamoto A, Sato K, Wada T, Takahashi H, Iizuka H, Syringocystadenocarcinoma papilliferum: Case report and immuno histochemical comparison with its benign counterpartJ Am Acad Dermatol 2001 45:755-59.10.1067/mjd.2001.11772311606929 [Google Scholar] [CrossRef] [PubMed]
[9]. Abrari A, Mukherjee U, Syringocystadenocarcinoma papilliferum at unusual site: Inherent lesional histologic polymorphism is the pathognomonBMJ Case Rep 2011 10.1136/bcr.05.2011.425410.1136/bcr.05.2011.425422693305 [Google Scholar] [CrossRef] [CrossRef] [PubMed]
[10]. Peterson J, Tefft K, Blackmon J, Rajpara A, Fraga G, Syringocystadenocarcinoma papilliferum: A rare tumor with a favorable prognosisDermatology Online Journal 2013 19:11 [Google Scholar]
[11]. Iga N, Fujii H, Miyake T, Ehara M, Kore-eda S, Syringocystadenocarcioma papilliferum in the perianal areaCase Rep Dermatol 2015 7:84-89.10.1159/00038194026078737 [Google Scholar] [CrossRef] [PubMed]
[12]. Calonje E, Brenn T, Lazar A, McKee PH, Tumors of the sweat glandsIn: McKee’s Pathology of the Skin with Clinical Correlations 2012 4th edEdinburghElsevier:151210.1016/B978-1-4160-5649-2.00033-0 [Google Scholar] [CrossRef]
[13]. Tuffaha MSA, Guski H, Kristiansen G, Markers and Immunoprofile of Skin TumorsIn: Immunohistochemistry in Tumor Diagnostics 2018 SwitzerlandSpringer International Publishing:191-3.10.1007/978-3-319-53577-7_20 [Google Scholar] [CrossRef]
[14]. Weedon D, Strutton G, Rubin AI, Tumors of cutaneous appendages. In: Weedon D. editorWeedon’s skin pathology 2011 3rd edEdinburghElsevier:781 [Google Scholar]
[15]. Dissanayake RV, Salm R, Sweat-gland carcinoma. Prognosis related to histological typeHistopathology 1980 4:445-66.10.1111/j.1365-2559.1980.tb02939.x6253380 [Google Scholar] [CrossRef] [PubMed]
[16]. Seco Navedo MA, Fresno Forcelledo M, Orduna Domingo A, Junco Petrement P, Soler Sanchez T, Syringocystadenoma papilliferum with malignant evolution: Presentation of a caseAnn Dermatol Venerol 1982 109:685-89. [Google Scholar]
[17]. Numata M, Hosoe S, Itoh N, Munakata Y, Hayashi S, Maruyama Y, Syringoadenocarcinoma papilliferumJ Cutan Pathol 1985 12:03-07.10.1111/j.1600-0560.1985.tb00422.x [Google Scholar] [CrossRef]
[18]. Bondi R, Urso C, Syringocystadenocarcinoma papilliferumHistopathology 1996 28:475-77.10.1046/j.1365-2559.1996.t01-4-297345.x8735727 [Google Scholar] [CrossRef] [PubMed]
[19]. Arai Y, Kusakabe H, Kiyokane K, A case of syringocystadenocarcinoma papilliferum in situ occurring partially in syringocystadenoma papilliferumJ Dermatol 2003 30:146-50.10.1111/j.1346-8138.2003.tb00363.x12692383 [Google Scholar] [CrossRef] [PubMed]
[20]. Chi CC, Tsai RY, Wang SH, Syringocystadenocarcinoma papilliferum: Sucessfully treated with Mohs microscopic surgeryDermatol Surg 2004 30:468-71.10.1111/j.1524-4725.2004.30023.x15008887 [Google Scholar] [CrossRef] [PubMed]
[21]. Wostenborghs H, Van Evkev P, Dans A, Syringocystadenocarcinoma papilliferum in situ with pagetoid spread: A case reportHistopathology 2006 48:869-70.10.1111/j.1365-2559.2006.02421.x16722938 [Google Scholar] [CrossRef] [PubMed]
[22]. Langner C, Ott A, Syringocystadenocarcinoma papilloma in situoriginating from the perianal skinAPMIS 2009 117:148-50.10.1111/j.1600-0463.2008.00027.x19239438 [Google Scholar] [CrossRef] [PubMed]
[23]. Sroa N, Sroa N, Zirwas M, Syringocystadenocarcinoma papilliferumDermatol Surg 2010 36:261-63.10.1111/j.1524-4725.2009.01403.x20002633 [Google Scholar] [CrossRef] [PubMed]
[24]. Hoekzema R, Leenarts MF, Nijhuis EW, Syringocystadenocarcinoma papilliferum in a linear nevus verrucosusJ Cutan Pathol 2011 38:246-50.10.1111/j.1600-0560.2009.01419.x19758371 [Google Scholar] [CrossRef] [PubMed]