Exophiala jeanselmei and Rhizopus oryzae Co-infection Post Renal Transplant
Suneeta Meena1, Gagandeep Singh2, Mragnayani Pandey3, Immaculata Xess4
1 Senior Resident, Department of Microbiology, AIIMS, Delhi, India.
2 Assistant Professor, Department of Microbiology, AIIMS, Delhi, India.
3 PhD Scholar, Department of Microbiology, AIIMS, Delhi, India.
4 Professor, Department of Microbiology, AIIMS, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Immaculata Xess, Professor, Department of Microbiology, AIIMS, Delhi, India.
E-mail: immaxess@gmail.com
Invasive fungal infections are associated with morbidity and mortality in immunocompromised patients. Dematiaceous fungi are being increasingly recognised as human pathogens, particularly in transplant recipients. However, most cases of dematiaceous fungal infections after organ transplantation have been reported as sole aetiological agents. We report a case of fatal, disseminated phaeohyphomycosis with rhinocerebral mucormycosis in post renal transplant patient. Quick diagnosis is challenging and inspite of aggressive management with a combination of surgical and medical therapies, the outcome is poor. Amphotericin B remains the cornerstone in the medical management of mucormycosis and itraconazole in phaeohyphomycosis, still we are short of drugs to fight against such catastrophic organisms.
Dematiaceous fungi, Mucormycosis, Phaeohyphomycosis
Case Report
A 45-year-old farmer presented with chief complaint of facial swelling, decreased vision and fever since two weeks. On physical examination patient was febrile with left orbital swelling and blackish brown mucus draining from the sinuses. The patient had no chills, cough, or sputum production. He had a single, solid, painful, pigmented subcutaneous nodule on left ankle [Table/Fig-1].
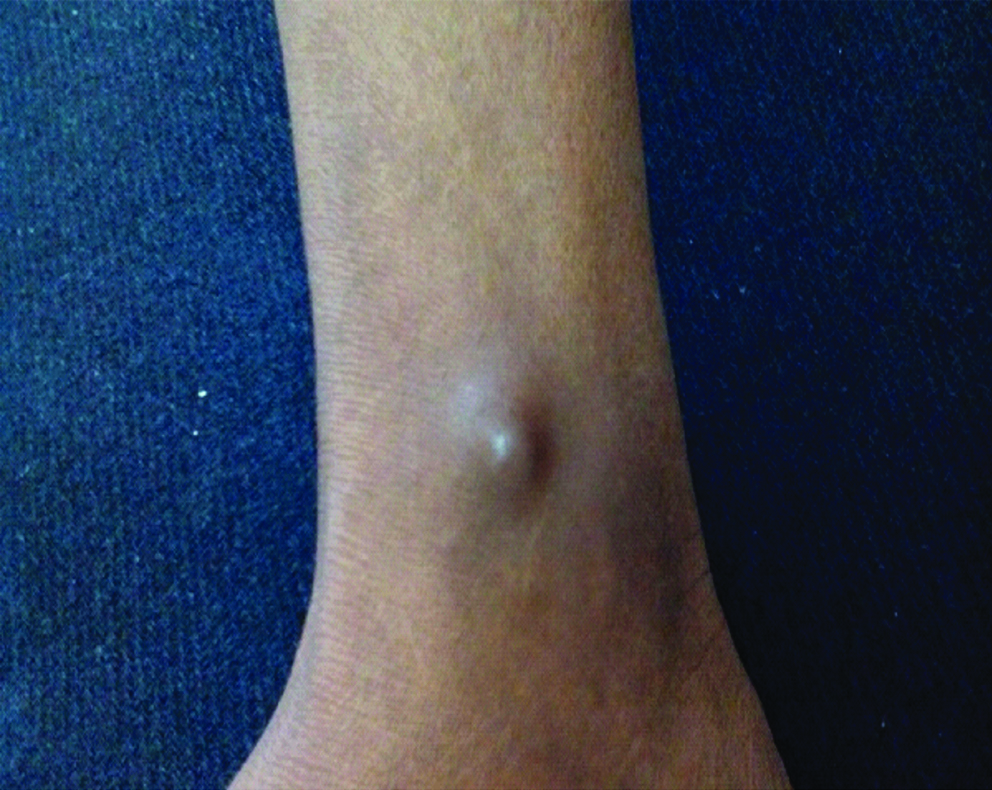
The patient had a history of diabetic nephropathy, for which he underwent cadaveric renal transplant in March 2006 (12 years back). His postoperative period was uneventful. His baseline creatinine serum level was 1.5 mg/L. At the time of admission, his fasting blood sugar was 300 mg/dL, but there was no ketoacidosis. Other laboratory tests including urinalysis, complete blood count, liver enzymes, and serum billirubin were normal. His current immunosuppressive therapy was cyclosporine 50 mg two times a day, azathioprine 50 mg/d and prednisolone 5 mg/d.
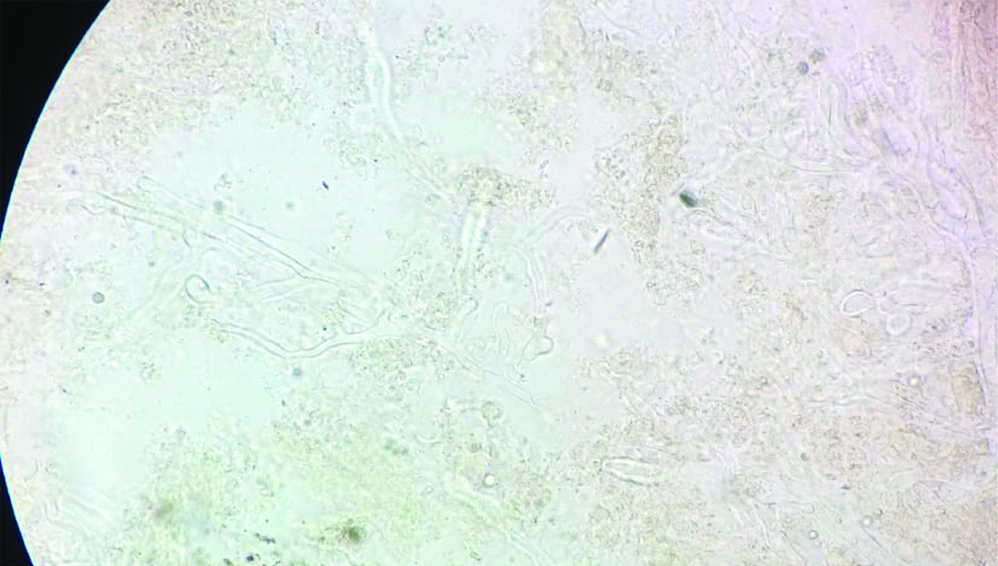
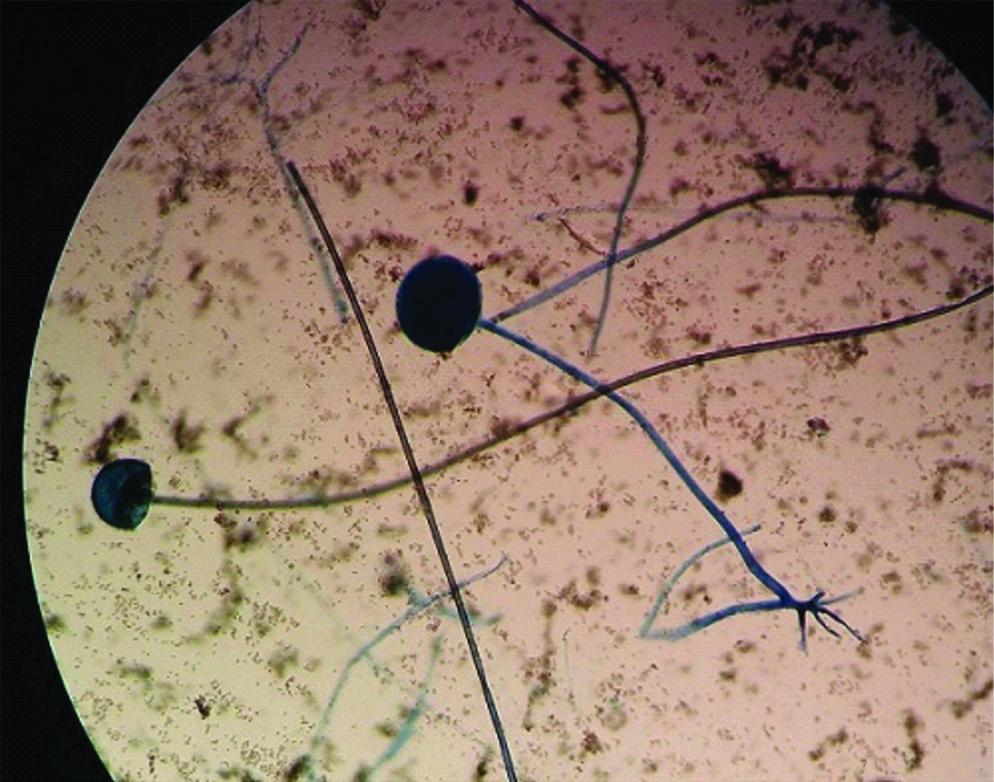
On day 1 of admission Computed Tomography (CT) scan of the sinuses showed opacification of the left maxillary, ethmoidal, and sphenoidal sinuses. Therefore, he had to undergo Functional Endoscopic Sinus (FESS) surgery. Aspirate from the sinuses was sent to mycology laboratory where broad aseptate hyphae with wide angled branching were seen in 10% potassium hydroxide (KOH) mount [Table/Fig-2]. His immunosuppressive drugs were discontinued, and liposomal amphotericin B, 50 mg/d, was started, which gradually increased up to 200 mg/d within 3 days. The patient underwent aggressive surgical debridement of the sinuses. Simultaneously, fine needle aspirate from a subcutaneous nodule on left leg, was sent to mycology laboratory where dematiaceous septate hyphae were seen in 10% KOH mount. Also, in view of persistence of fever spikes blood culture was sent which yielded black fungal growth on two separate blood cultures drawn a week apart. On day 18, patient developed progressive worsening of symptoms with persistent fever, deteriorating renal function and severe metabolic acidosis. Addition of voriconazole was planned, but before any other antifungal could be started in view of mixed fungal infection patient suddenly developed refractory shock and ultimately succumbed to death. Fungal infection disseminated very rapidly and co-infection further contributed to the cause. Diagnosis of rhinocerebral mucormycosis due to Rhizopus oryzae and Exophiala jeanselmei fungemia post renal transplant was established.
KOH mount of FESS aspirate from sinuses (400X).
Mycology Examination
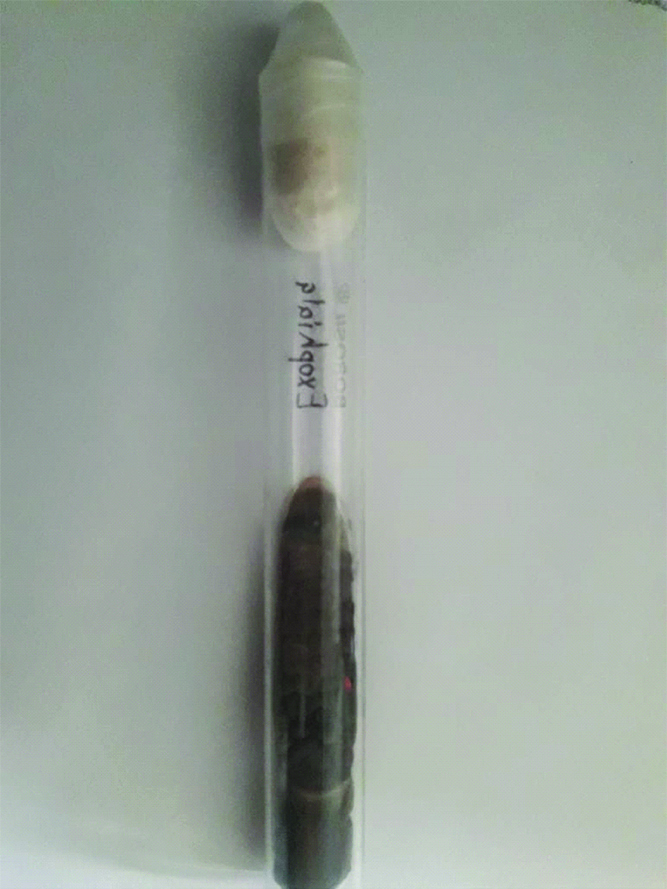
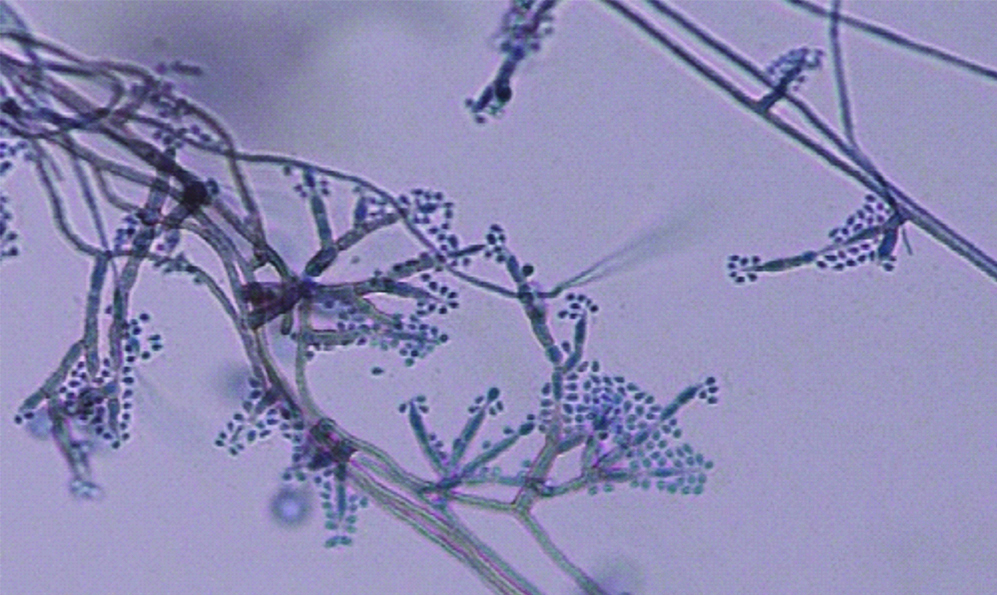
Blood culture as well as fine needle aspirate from subcutaneous nodule yielded a black mycelial growth after a week of incubation [Table/Fig-3]. The isolate was inoculated on to Potato Dextrose Agar (PDA) and incubated at both 25°C and 37°C for better recovery of the fungus in suspicion of dimorphic fungus. All tubes on to PDA grew black colonies with olivaceous black reverse on the seventh day of incubation at both temperatures. Lactophenol Cotton Blue Mount (LPCB) showed numerous dematiaceous septate hyphae with conidiophores bearing ellipsoidal annelloconidia suggestive of Exophiala species [Table/Fig-4]. Slide culture was put up on cornmeal agar. On the basis of morphology isolate was identified as Exophiala jeanselmei. To confirm the identity of the isolate, Internal Transcribed Spacer (ITS) regions (ITS1-5.8S-ITS2) were amplified using ITS1 (5′-TCCGTAGGTGAACCTGCGG-3) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) as described previously [1]. Comparison of the nucleotide sequence of our isolate with Gene Bank database using Basic Local Alignment Search Tool (BLAST) algorithm yielded 100% homology with Exophiala jeanselmei reference strain KX218394.
Culture of Exophiala jeanselmei.
LPCB mount of Exophiala jeanselmei (400X).
Culture of the FESS aspirate yielded a greyish-white floccose, dense and wooly colonies having hairy appearance within 72 hours of incubation and turning dark brown to black after 10-12 days of incubation at both 25°C and 37°C on saboraud’s dextrose agar [Table/Fig-5]. Lactophenol Cotton Blue (LPCB) preparations of the growth confirmed the presence of aseptate hyphae that were irregularly branched at right angles. Sporangiophores and sporangium containing sporangiospores were seen. Few vegetative, non-septate hyphae also revealed the presence of rhizoids arising beneath the regions where sporangiophores were rising. Morphologically the isolate was identified as Rhizopus oryzae [Table/Fig-6]. It was subsequently sequenced for speciation using similar primers as used for Exophiala jeanselmei. Comparison of the nucleotide sequence of our isolate with Gene Bank database using BLAST algorithm yielded 100% homology with Rhizopus oryzae reference strain KX237566.
Culture of Rhizopus oryzae.

LPCB mount of Rhizopus oryzae (400X).
Final diagnosis of mixed fungal infection, rhino cerebral mucormycosis due to Rhizopus oryzae and fungemia due to Exophiala jeanselmei was made.
Discussion
Mixed fungal infections in renal transplant recipients have been reported previously mostly in early post-transplant [2]. But, in this case patient acquired infection in late period 12 years post-transplantation. The symptoms of systemic fungal infections are nonspecific, particularly in their early stages [3]. However, in this patient rhinocerebral mucormycosis ended up masking phaeohyphomycosis due to Exophiala jeanselmei which was isolated both from subcutaneous nodule and blood after eight days of admission delaying the diagnosis. Rhinocerebral mucormycosis itself has a very rapid course of progression in diabetic patients but additional immunocompromised status due to renal transplant may have predisposed the patient to acquiring Exophiala jeanselmei. Systemic mycosis due to this black fungus is a rare occurence so high index of suspicion is necessary. Fungal infections predominate in first six months post-transplant patients due to severe immunocompromised status [4]. But in this case patient acquired infection after 12 years of transplant.
Treatment with amphotericin B remains the gold standard of systemic antifungal therapy but addition of triazole drugs has been attempted in cases of co-infection. Broad spectrum triazole antifungal agents that show good activity against clinically important yeasts and molds including dematiaceous fungi should be preferred in case of co-infection. Best possible treatment for Exophiala spp is still not established, although itraconazole, posaconazole with surgical excision has been reported to have good clinical result [5,6]. The recent European Society of Clinical Microbiology and Infectious Diseases and European Confederation of Medical Mycology (ESCMID/ECMM) joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis, suggest oral itraconazole being the drug of choice, given the extensive clinical experience [7]. Previously, all voriconazole, itraconazole, posaconazole and amphotericin B have shown antifungal activity against Exophiala spp but recent studies hints that antifungal activity differs between species [8]. This case also highlights the importance of using immunosuppressors with minimal drug interaction during post-transplant infection. Itraconazole is known to inhibit metabolism of corticosteroids [9]. Many chemotherapeutic agents rely on the CYP enzyme system for activation and/or metabolism. Data are limited on interactions between chemotherapeutic agents and azole antifungals; however, several of the azole agents may require dosage adjustments or interruptions in therapy when immunosuppressive treatments are administered [10]. Hence, limiting the options of antifungals that can be started empirically and also, making it necessary to speciate organism for optimal treatment.
Exophiala jeanselmei has been reported previously in causing subcutaneous phaeohyphomycosis in patients post renal transplant. It may cause pleomorphic infections ranging from superficial subcutaneous infections to fatal disseminated diseases [11]. Rhinocerebral mucormycosis due to mixed fungi has been reported previously in post renal transplant but, to the best of our knowledge this is the first case of co-infection with black fungus involving a different site.
Conclusion
Fungal infections pose difficult diagnostic and therapeutic challenges. Rapid course of fungal infection require early diagnosis and treatment to increase the likelihood of survival in an immunosuppressed patient. Our case points to the necessity to use rapid, sensitive and specific molecular methods for identification of fungal pathogens in clinical settings.
[1]. White TJ, Bruns TD, Lee SB, Taylor JW, PCR-Protocols and Applications-A Laboratory Manual 1990 New YorkAcademic PressAmplification and sequencing of fungal ribosomal RNA genes for phylogenetics; Pp. 315-32210.1016/B978-0-12-372180-8.50042-11696192 [Google Scholar] [CrossRef] [PubMed]
[2]. Kubak BM, Huprikar SS, AST Infectious Diseases Community of PracticeEmerging & rare fungal infections in solid organ transplant recipientsAm J Transplant 2009 9(Suppl 4):S208-26.10.1111/j.1600-6143.2009.02913.x20070683 [Google Scholar] [CrossRef] [PubMed]
[3]. Chugh KS, Sakhuja V, Jain S, Singh V, Tarafdar A, Joshi K, Fungal infections in renal allograft recipientsTransplant Proc 1992 24(5):1940-42. [Google Scholar]
[4]. Patel R, Paya CV, Infections in solid-organ transplant recipientsClin Microbiol Rev 1997 10(1):86-124.10.1128/CMR.10.1.868993860 [Google Scholar] [CrossRef] [PubMed]
[5]. Al-Tawfiq JA, Amr SS, Madura leg due to Exophiala jeanselmei successfully treated with surgery and itraconazole therapyMed Mycol 2009 47(6):648-52.10.1080/1369378080266919419145516 [Google Scholar] [CrossRef] [PubMed]
[6]. Negroni R, Helou SH, Petri N, Robles AM, Arechavala A, Bianchi MH, Case study: Posaconazole treatment of disseminated phaeohyphomycosis due to Exophiala spiniferaClin Infect Dis 2004 38(3):15-20.10.1086/38084014727230 [Google Scholar] [CrossRef] [PubMed]
[7]. Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, Arendrup MC, European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungiClin Microbiol Infect 2014 20(Suppl 3):47-75.10.1111/1469-0691.1256924606200 [Google Scholar] [CrossRef] [PubMed]
[8]. Fothergill AW, Rinaldi MG, Sutton DA, Antifungal susceptibility testing of Exophiala spp.: a head-to-head comparison of amphotericin B, itraconazole, posaconazole and voriconazoleMed Mycol 2009 47(1):41-43.10.1080/1369378080251245119107637 [Google Scholar] [CrossRef] [PubMed]
[9]. Moriyama B, Henning SA, Leung J, Falade-Nwulia O, Jarosinski P, Penzak SR, Adverse interactions between antifungal azoles and vincristine: Review and analysis of casesMycoses 2012 55(4):290-97.10.1111/j.1439-0507.2011.02158.x22126626 [Google Scholar] [CrossRef] [PubMed]
[10]. Lebrun-Vignes B, Archer VC, Diquet B, Levron JC, Chosidow O, Puech AJ, Effect of itraconazole on the pharmacokinetics of prednisolone and methylprednisolone and cortisol secretion in healthy subjectsBr J Clin Pharmacol 2001 51(5):443-50.10.1046/j.1365-2125.2001.01372.x11422002 [Google Scholar] [CrossRef] [PubMed]
[11]. Sayilar EI, Ersoy A, Ayar Y, Akalin H, Ceylon I, Girgin NK, Mixed fungal infection in early period after kidney transplantation: a case reportTurkish Nephrology Dialysis Transplantation 2015 24:134-37.10.5262/tndt.2015.1001.24 [Google Scholar] [CrossRef]