Introduction
Association of serum folate, Vitamin B6 and B12 deficiencies with hyperhomocysteinemia are well-documented. Several studies on Indian population, address the association of hyperhomocysteinemia with numerous diseases, but population-specific baseline levels of homocysteine in healthy population have not been elucidated till date.
Aim
To establish Indian-population specific reference ranges for total plasma homocysteine in healthy adults in accordance to variation in dietary factors, serum B12 and folate status. The study also aimed to assess the contribution of cofactors in modulating homocysteine levels, and to evaluate the impact of higher homocysteine levels on the oxidative DNA damage.
Materials and Methods
In this retrospective study, 151 apparently healthy non-diabetic, non-hypertensive individuals with normal thyroid, renal and cardiac function were recruited. Vitamin B12, Folate, and Homocysteine assays were performed on ADVIA Centaur® XP Immunoassay system. Plasma 8-oxo-2deoxyguanosine levels were estimated by using commercially available ELISA kit. Student’s t-test, ANOVA, Spearman rank correlation was used to establish the associations between variables. The p<0.05 was considered statistically significant.
Results
The data was stratified according to age (Group 1: ≤45 yr, n=58 and Group 2: >45 yr, n=93) and gender. In both the age groups, homocysteine and 8-oxo-2deoxyguanosine levels were found to be elevated in men (p=0.003) while no gender-specific differences were observed in the levels of folate and B12. Post-menopausal women had higher oxidative DNA damage as compared to the pre-menopausal women. Positive correlation was observed between total plasma homocysteine and plasma 8-oxo-2deoxyguanosine (r=0.35, p=0.03). An inverse correlation was observed between plasma B12 levels and total plasma homocysteine in men (r=-0.24, p=0.02) while no such correlation was observed in women (r=0.22, p=0.10). No significant correlation was observed between plasma folate levels and total plasma homocysteine in both genders (male: r=-0.10, p=0.33; female: r=-0.23, p=0.09). Non-vegetarians had higher plasma B12 levels (p<0.05) and lower homocysteine levels (p=0.03) than vegetarians.
Conclusion
Healthy Indian adults have higher homocysteine levels than their counterparts due to vegetarian diet resulting in B12 deficiency. A positive correlation was observed between total plasma homocysteine and oxidative DNA damage. Higher oxidative DNA damage was observed in post-menopausal women than pre-menopausal women, which might predispose them to many multifactorial disorders.
Introduction
One-carbon metabolic pathway plays a crucial role in synthesis, repair and methylation of DNA thus perturbations in this pathway are associated with multiple diseases [1]. Homocysteine serves as a surrogate marker of perturbations in the re-methylation of homocysteine to methionine and transsulfuration of homocysteine to cysteine through cystathione. Defect in re-methylation process is accompanied by impaired synthesis of S-adenosyl methionine thus affects the cellular methylation status [2]. Defect in transsulfuration process is accompanied by elevation of S-adenosyl homocysteine and lowered cysteine levels [3]. Lower levels of cysteine were reported to be associated with reduced levels of glutathione in liver and skeletal muscles [4]. The re-methylation process requires 5-methyl tetrahydrofolate as the substrate, methyltetrahydrofolate homocysteine Methyltransferase (MTR) as the catalyst and methylcobalamin as the cofactor. The reductive methylation of cobalamin is catalyzed by methyltetrahydrofolate homocysteine Methyltransferase Reductase (MTRR) [5]. Cystathionine β-synthase is the first enzyme executing the transsulfuration pathway, which is allosterically activated by S-adenosy lmethionine and uses pyridoxal phosphate as the cofactor for the conversion of homocysteine to cystathionine [6]. Cystathionine β-lyase catalyses cleavage of cystathionine to cysteine through β-elimination of homocysteine moiety [6]. Several putatively functional polymorphisms were reported in the genes regulating one-carbon metabolism, which influence homocysteine levels [7,8]. Apart from genetic contributors, deficiencies of folate, B6 and B12 are also associated with hyperhomocysteinemia [9].
In the year 1997, the incidences of anemia, B6 deficiency and folate deficiency among Indian women were reported to be 15%, 84% and 24.5%, respectively [10]. High prevalence of cobalamin deficiency (47%) and moderate prevalence of folate deficiency (5%) were reported among Asian Indians [11]. A study comprising of 40 normal men from South India reported 19.83±1.25 μmol/L as the mean homocysteine with RBC and plasma folate showing inverse association with homocysteine [12]. Various studies on Indian population suggest association of hyperhomocysteinemia with multiple diseases [13-15]. Population-specific baseline levels of homocysteine in relatively healthy population have not been elucidated till date. Only one study established reference ranges of homocysteine in Indian newborns and no such studies are available on the adult population [16]. At present, reference ranges for homocysteine in Indian population are based on western data. In view of variations in dietary factors, B12 and folate status, genetic and environmental factors, reference ranges established from the Western population cannot be used to predict Indian scenario.
Therefore, the rationale of the current study was to establish Indian-population specific reference ranges for total plasma homocysteine in healthy adults, to elucidate the contribution of folate and B12 levels in modulating homocysteine; and to assess the impact of elevated homocysteine levels on the oxidative DNA damage.
Materials and Methods
The retrospective study was conducted from June 2018 to January 2019 in the Outpatient Department of Nizam’s Institute of Medical Sciences, Hyderabad, India. This study was approved from the Institutional ethics committee (EC/NIMS/1931/2017) of Nizam’s Institute of Medical Sciences, Hyderabad, India. A total of 151 healthy controls (95 men and 56 women) above 20 years of age who were non-diabetic, non-hypertensive and had normal thyroid, renal and cardiac function, were enrolled after obtaining an informed written consent. The study participants included institutional staff and volunteers accompanying the patients. Subjects taking S-adenosyl-methionine, methotrexate, nicotinic acid, theophylline, nitrous oxide, or L-dopa were excluded from the study.
About 5 mL of fasting venous blood sample was collected in EDTA anticoagulant tube. Plasma was obtained by centrifugation at 2000 × g for 10 min at room temperature. Homocysteine level was estimated immediately after plasma collection, and one plasma aliquot of each sample was stored at -20°C until 8-oxo-2deoxyguanosine measurement. Vitamin B12, Folate, and Homocysteine assays were performed on ADVIA Centaur® XP Immunoassay system using kits manufactured by Simens Healthcare Diagnostics Inc., USA. Plasma 8-oxo-2deoxyguanosine levels were estimated by using commercially available ELISA kit (Cat. No. ab201734) obtained from Abcam, MA, USA. All the estimations were performed as per manufacture’s manual.
Statistical Analysis
The unpaired student’s t-test was used for assessing mean differences in continuous variables between two groups. Analysis of variance (ANOVA) was used when groups were more than two. Spearman rank correlation coefficient was used as index of association between two variables. For all the statistical analysis, p<0.05 was considered statistically significant.
Results
The biochemical parameters namely homocysteine, folate, B12 and 8-oxo-2-deoxyguanosine were studied and segregated based on age and gender. The homocysteine data was stratified in two groups based on age i.e., Group 1: 20-45 years (n=58) and Group 2: >45 years (n=93) and the mean age of this cohort was 49.8±13.3 years. The plasma homocysteine levels in males were higher than females (20.7±9.2 vs. 15.5±5.8 μmol/L) irrespective of the age [Table/Fig-1].
Distribution of biochemical variables among the study cohort.
| Variable | Mean±standard deviation | p-value |
|---|
| Men (n=95) | Women (n=56) |
|---|
| Homocysteine (μmol/L) |
| Group 1 | 19.2±6.3 | 14.3±2.8 | 0.003* |
| Group 2 | 21.9±10.7 | 16.0±6.7 | 0.003* |
| B12 (pg/mL) |
| Group 1 | 378±232 | 398±195 | 0.80 |
| Group 2 | 454±208 | 466±190 | 0.69 |
| Folate (ng/mL) |
| Group 1 | 11.4±8.5 | 11.1±10.4 | 0.91 |
| Group 2 | 10.3±6.1 | 9.5±7.3 | 0.56 |
| 8-oxo-2-deoxyguanosine (ng/mL) |
| Group 1 | 6.1±3.7 | 4.7±2.0 | <0.05* |
| Group 2 | 8.9±3.7 | 6.7±4.2 | 0.04* |
Group 1: ≤45 years age group: Group 2: >45 years age group. ANOVA test *p<0.05 statistically significant
An inverse correlation was observed between plasma B12 levels and total plasma homocysteine in men (r=-0.24, p=0.02) while no such correlation was observed in women (r=0.22, p=0.10) [Table/Fig-2]. Plasma B12 levels were higher in non-vegetarians than vegetarians (444±254 pg/mL vs. 239±118 pg/mL, p=0.05).
Correlation of plasma B12 with homocysteine in men (a) and women (b).
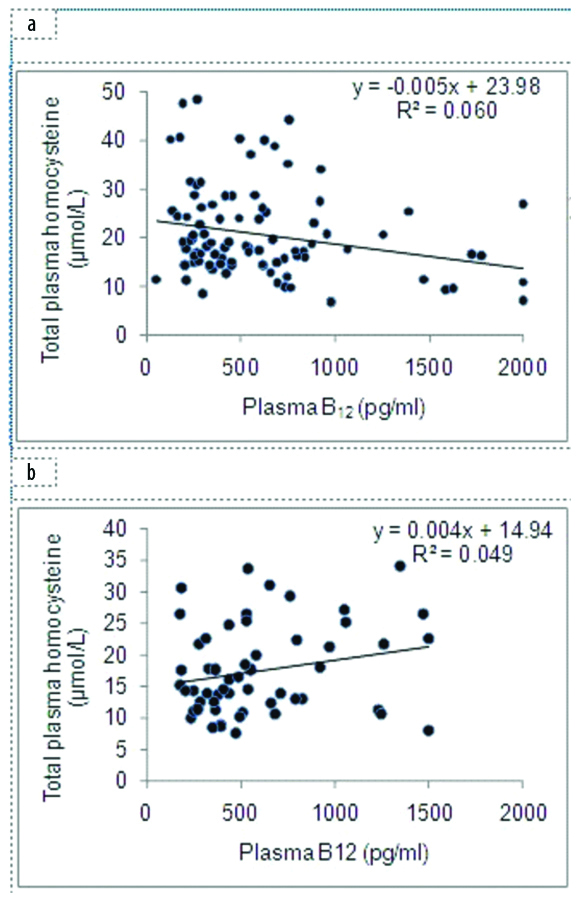
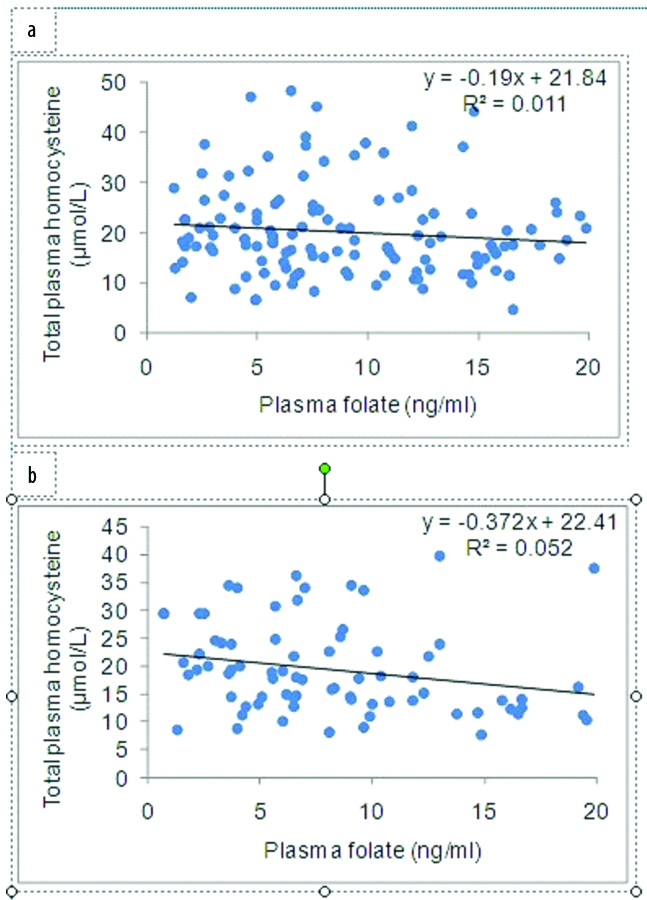
No significant correlation was observed between plasma folate levels and total plasma homocysteine in both genders (male: r=-0.10, p=0.33; female: r=-0.23, p=0.09) [Table/Fig-3].
Correlation of plasma folate with homocysteine in men (a) and women (b).
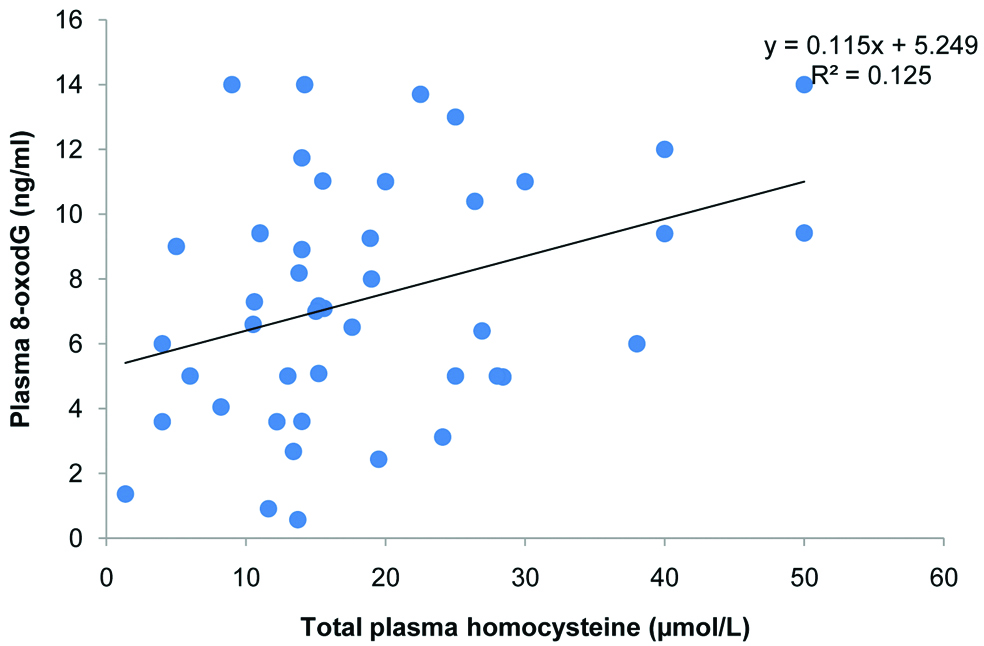
Homocysteine levels were observed to be lower in non-vegetarians compared to vegetarians. (13.1±6.5 μmol/L vs. 20.9±18.9 μmol/L, p=0.03). A positive correlation was observed between total plasma homocysteine and plasma 8-oxo-2deoxyguanosine (r=0.35, p=0.03) [Table/Fig-4].
Correlation of total plasma homocysteine with plasma 8-oxodG.
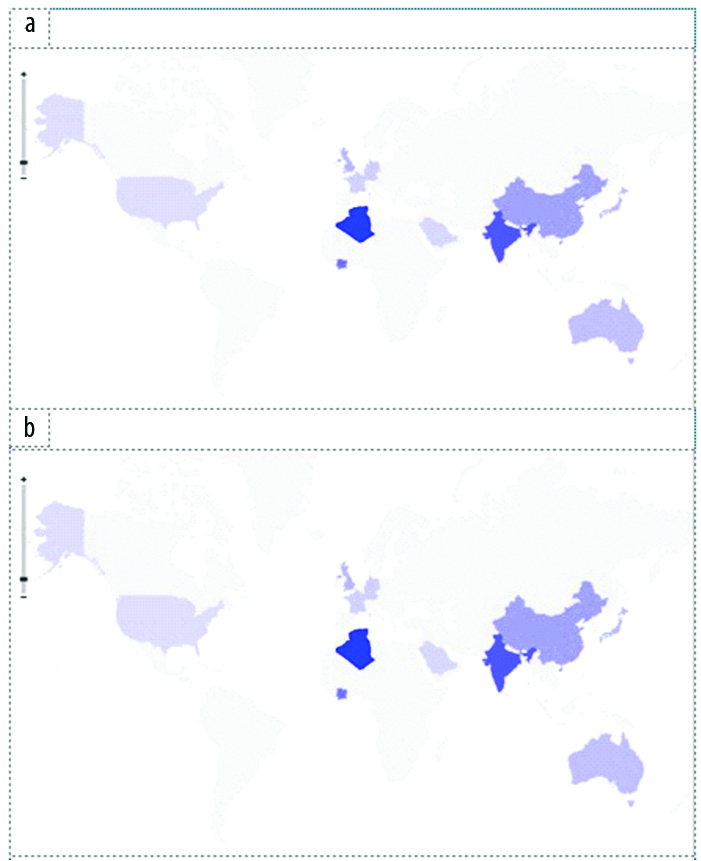
A literature-based comparison with ten studies across the globe on homocysteine levels in adult population revealed that homocysteine levels were higher in Indian, Algerian and Ivorian populations [Table/Fig-5] [17-25].
Population-specific distribution of homocysteine in men (a) and women (b) [17-25].
Discussion
The current study established baseline reference values of homocysteine for the adult Indian population. Exclusion of diabetes, hypertension, dyslipidemia and other co-morbid cardiac and renal conditions makes this data more authentic. In addition, the micronutrient status in terms of plasma folate and B12; impact of homocysteine elevation on oxidative DNA damage were measured. No gender differences were observed in the distribution of vitamin B12 and folate. Homocysteine levels were lower in women in both age groups. Women had lower levels 8-oxo-2-deoxyguanosine compared to men suggesting lower oxidative DNA damage in women. An inverse correlation was observed between homocysteine and vitamin B12 in men while no such correlation was observed in women. No statistically significant correlation was observed between plasma homocysteine and folate. The plasma homocysteine was reported to have positive correlation with fat free mass and testosterone and an inverse correlation with estradiol [20]. In the presence of adequate estradiol levels, homocysteine mediated oxidative DNA damage was reported to be quenched which is consistent with lower oxidative stress in women, specifically pre-menopausal women since post-menopausal women experience more oxidative DNA damage due to lower levels of estradiol [26].
Reduced homocysteine levels were observed in non-vegetarians compared to vegetarians. The plasma B12 levels were higher in non-vegetarians than vegetarians. Post-menopausal women had higher homocysteine levels and higher oxidative stress than pre-menopausal women. These results conclude that homocysteine levels are higher in Indian adults compared to their counterparts in other populations, which is attributed partly to vegetarian diet resulting in B12 deficiency. This observation is consistent with an earlier study, which reported higher homocysteine levels in vegans who avoid all foods of animal origin when compared to high meat eaters [27]. Apart from non-vegetarian diet, daily consumption of milk was reported to increase plasma B12 levels thus reducing homocysteine [28].
Apart from the dietary determinants of homocysteine, several genetic determinants were identified among which Methylene Tetrahydrofolate Reductase (MTHFR) C677T, 5-methyltetrahydrofolate homocysteine methyltransferase (MTR) A2756G and 5-methyltetrahydrofolate homocysteine methyltransferase reductase (MTRR) A66G were shown to be positively associated with homocysteine, while hydroxymethyltransferase 1 (SHMT1) C1420T and TYMS 5′-UTR 28 bp tandem repeat were reported to have negative association with homocysteine [29].
The present study has observed that the population-level differences in the distribution of homocysteine are consistent with a multi-ethnic study conducted in United states whereas in Asian Indians exhibited higher homocysteine levels compared to other ethnic groups [11]. It has been observed from the heatmap diagram of homocysteine developed for this study that Indians, Africans and certain Asian populations have higher homocysteine levels while those far from the equator have relatively less homocysteine levels [Table/Fig-5]. Hence, the environmental factors such as climate, cooking patterns and dietary habits might be influencing the variation in homocysteine levels globally. MTHFR, one of the major rate limiting enzyme of homocysteine metabolic pathway was found to be thermolabile in certain subjects who harbor MTHFR 677 C>T polymorphism, which induces enhanced propensity to dissociate into inactive monomers with loss in FAD-binding capacity. Apart from this, prolonged cooking habits result in loss of major nutrients such as folate and B12 from the diet. Vegetarian diet also is a major contributor to elevated homocysteine levels.
Despite the study being conducted in apparently healthy population, a positive association of homocysteine with oxidative DNA damage was observed. This is consistent with our previous study on breast cancer where in hyperhomocysteinemia was reported to induce BRCA1 methylation and thus affecting the formation of BRCA1-associated genome surveillance complex, which plays a pivotal role in the maintenance of genomic integrity [30].
To the best of our knowledge, this is the first study from India conducted exclusively on healthy adult population to establish baseline homocysteine reference ranges. The key dietary determinants such as plasma folate and B12, known to modulate homocysteine were included in the study. Induction of oxidative DNA damage was also observed in subjects with elevated homocysteine levels. However, future studies are warranted to investigate the clinical utility of folate or B12 supplementation in reducing homocysteine levels and decreasing oxidative DNA damage.
Limitation
The major limitation is lack of follow-up of subjects for possible evaluation. Further, no supplementation was advised as it was the initial study for assessing the true micronutrient status, and the contribution of genetic factors towards homocysteine could not be established.
Conclusion
The reference ranges of homocysteine in Indian adults are higher than their counterparts. The deficiency of B12 is likely contributor for this elevation, specifically in men. Regular consumption of meat was shown to lower homocysteine levels by correcting B12 deficiency. Homocysteine is positively correlated with oxidative DNA damage, which might increase the susceptibility towards geriatric disorders, including cardiovascular disorders and cancer.
Group 1: ≤45 years age group: Group 2: >45 years age group. ANOVA test *p<0.05 statistically significant
[1]. Naushad SM, Vijayalakshmi SV, Rupasree Y, Kumudini N, Sowganthika S, Naidu JV, Multifactor dimensionality reduction analysis to elucidate the cross-talk between one-carbon and xenobiotic metabolic pathways in multi-disease modelsMol Biol Rep 2015 42(7):1211-24.10.1007/s11033-015-3856-z25648260 [Google Scholar] [CrossRef] [PubMed]
[2]. Zhang N, Role of methionine on epigenetic modification of DNA methylation and gene expression in animalsAnim Nutr 2018 4(1):11-16.10.1016/j.aninu.2017.08.00930167479 [Google Scholar] [CrossRef] [PubMed]
[3]. Orendác M, Zeman J, Stabler SP, Allen RH, Kraus JP, Bodamer O, Homocystinuria due to cystathionine beta-synthase deficiency: Novel biochemical findings and treatment efficacyJ Inherit Metab Dis 2003 26(8):761-73.10.1023/B:BOLI.0000009963.88420.c214739681 [Google Scholar] [CrossRef] [PubMed]
[4]. Ishii I, Akahoshi N, Yamada H, Nakano S, Izumi T, Suematsu M, Cystathionine gamma-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injuryJ Biol Chem 2010 285(34):26358-68.10.1074/jbc.M110.14743920566639 [Google Scholar] [CrossRef] [PubMed]
[5]. Wolthers KR, Scrutton NS, Cobalamin uptake and reactivation occurs through specific protein interactions in the methionine synthase-methionine synthase reductase complexFEBS J 2009 276(7):1942-51.10.1111/j.1742-4658.2009.06919.x19243433 [Google Scholar] [CrossRef] [PubMed]
[6]. Renga B, Hydrogen sulfide generation in mammals: the molecular biology of cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE)Inflamm Allergy Drug Targets 2011 10(2):85-91.10.2174/18715281179477628621275900 [Google Scholar] [CrossRef] [PubMed]
[7]. Vinukonda G, Shaik Mohammad N, Md Nurul Jain J, Prasad Chintakindi K, Rama Devi Akella R, Genetic and environmental influences on total plasma homocysteine and Coronary Artery Disease (CAD) risk among South IndiansClin Chim Acta 2009 405(1-2):127-31.10.1016/j.cca.2009.04.01519394322 [Google Scholar] [CrossRef] [PubMed]
[8]. Paré G, Chasman DI, Parker AN, Zee RR, Mälarstig A, Seedorf U, Novel associations of CPS1, MUT, NOX4, and DPEP1 with plasma homocysteine in a healthy population: a genome-wide evaluation of 13 974 participants in the Women’s Genome Health StudyCirc Cardiovasc Genet 2009 2(2):142-50.10.1161/CIRCGENETICS.108.82980420031578 [Google Scholar] [CrossRef] [PubMed]
[9]. Chen SF, Cui CL, Wu P, Xie NZ, Relationship of serum homocysteine level with nutritional status and HbA1c level in elderly inpatientsInt J Clin Exp Med 2013 6(9):779-84. [Google Scholar]
[10]. Neela J, Raman L, The relationship between maternal nutritional status and spontaneous abortionNatl Med J India 1997 10(1):15-16. [Google Scholar]
[11]. Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian IndiansAm J Clin Nutr 2001 74(2):233-41.10.1093/ajcn/74.2.23311470726 [Google Scholar] [CrossRef] [PubMed]
[12]. Lakshmi AV, Maniprabha C, Krishna TP, Plasma homocysteine level in relation to folate and vitamin B6 status in apparently normal menAsia Pac J Clin Nutr 2001 10(3):194-96.10.1046/j.1440-6047.2001.00252.x11708307 [Google Scholar] [CrossRef] [PubMed]
[13]. Kumar KS, Govindaiah V, Naushad SE, Devi RR, Jyothy A, Plasma homocysteine levels correlated to interactions between folate status and methylene tetrahydrofolate reductase gene mutation in women with unexplained recurrent pregnancy lossJ Obstet Gynaecol 2003 23(1):55-58.10.1080/014436102100004326312623486 [Google Scholar] [CrossRef] [PubMed]
[14]. Naushad S, Jamal NJ, Angalena R, Prasad CK, Devi AR, Hyperhomocysteinemia and the compound heterozygous state for methylene tetrahydrofolate reductase are independent risk factors for deep vein thrombosis among South IndiansBlood Coagul Fibrinolysis 2007 18(2):113-17.10.1097/MBC.0b013e3280108e0117287626 [Google Scholar] [CrossRef] [PubMed]
[15]. Kumudini N, Uma A, Naushad SM, Mridula R, Borgohain R, Kutala VK, Association of seven functional polymorphisms of one-carbon metabolic pathway with total plasma homocysteine levels and susceptibility to Parkinson’s disease among South IndiansNeurosci Lett 2014 568:1-5.10.1016/j.neulet.2014.03.04424686188 [Google Scholar] [CrossRef] [PubMed]
[16]. Radha Rama Devi A, Naushad SM, Prasad KC, Evaluation of total plasma homocysteine in Indian newborns using heel-prick samplesIndian J Pediatr 2006 73(6):503-08.10.1007/BF0275989516816512 [Google Scholar] [CrossRef] [PubMed]
[17]. Ni J, Zhang L, Zhou T, Xu WJ, Xue JL, Cao N, Association between the MTHFR C677T polymorphism, blood folate and vitamin B12 deficiency, and elevated serum total homocysteine in healthy individuals in Yunnan Province, ChinaJ Chin Med Assoc 2017 80(3):147-53.10.1016/j.jcma.2016.07.00528094233 [Google Scholar] [CrossRef] [PubMed]
[18]. Hambaba L, Abdessemed S, Yahia M, Laroui S, Rouabah F, Relationship between hyperhomocysteinemia and C677T polymorphism of methylene tetrahydrofolate reductase gene in a healthy Algerian populationAnn Biol Clin (Paris) 2008 66(6):637-41. [Google Scholar]
[19]. Devlin AM, Clarke R, Birks J, Evans JG, Halsted CH, Interactions among polymorphisms in folate-metabolizing genes and serum total homocysteine concentrations in a healthy elderly populationAm J Clin Nutr 2006 83(3):708-13.10.1093/ajcn.83.3.70816522921 [Google Scholar] [CrossRef] [PubMed]
[20]. Dierkes J, Jeckel A, Ambrosch A, Westphal S, Luley C, Boeing H, Factors explaining the difference of total homocysteine between men and women in the European Investigation Into Cancer and Nutrition Potsdam studyMetabolism 2001 50(6):640-45.10.1053/meta.2001.2328611398138 [Google Scholar] [CrossRef] [PubMed]
[21]. Nakazato M, Maeda T, Takamura N, Wada M, Yamasaki H, Johnston KE, Relation of body mass index to blood folate and total homocysteine concentrations in Japanese adultsEur J Nutr 2011 50(7):581-85.10.1007/s00394-010-0165-021221977 [Google Scholar] [CrossRef] [PubMed]
[22]. Silberberg J, Crooks R, Fryer J, Wlodarczyk J, Nair B, Finucane P, Fasting and post-methionine homocyst(e)ine levels in a healthy Australian populationAust N Z J Med 1997 27(1):35-39.10.1111/j.1445-5994.1997.tb00911.x9079251 [Google Scholar] [CrossRef] [PubMed]
[23]. Tiahou G, Dupuy AM, Jaussent I, Sees D, Cristol JP, Badiou S, Determinants of homocysteine levels in Ivorian rural populationInt J Vitam Nutr Res 2009 79(5-6):319-27.10.1024/0300-9831.79.56.31920533218 [Google Scholar] [CrossRef] [PubMed]
[24]. Carmel R, Mallidi PV, Vinarskiy S, Brar S, Frouhar Z, Hyperhomocysteinemia and cobalamin deficiency in young Asian Indians in the United StatesAm J Hematol 2002 70(2):107-14.10.1002/ajh.1009312111783 [Google Scholar] [CrossRef] [PubMed]
[25]. Ardawi MS, Rouzi AA, Qari MH, Dahlawi FM, Al-Raddadi RM, Influence of age, sex, folate and vitamin B12 status on plasma homocysteine in SaudisSaudi Med J 2002 23(8):959-68. [Google Scholar]
[26]. Mohan IK, Khan SA, Jacob R, Sushma Chander N, Hussain T, Alrokayan SA, Application of adaptive neuro-fuzzy inference systems (ANFIS) to delineate estradiol, glutathione and homocysteine interactionsClin Nutr ESPEN 2017 20:41-46.10.1016/j.clnesp.2017.03.00729072168 [Google Scholar] [CrossRef] [PubMed]
[27]. Herrmann W, Schorr H, Purschwitz K, Rassoul F, Richter V, Total homocysteine, vitamin B(12), and total antioxidant status in vegetariansClin Chem 2001 47(6):1094-101. [Google Scholar]
[28]. Naik S, Bhide V, Babhulkar A, Mahalle N, Parab S, Thakre R, Daily milk intake improves vitamin B-12 status in young vegetarian Indians: An intervention trialNutr J 2013 12(9):13610.1186/1475-2891-12-13624107225 [Google Scholar] [CrossRef] [PubMed]
[29]. Naushad SM, Rama Devi AR, Nivetha S, Lakshmitha G, Stanley AB, Hussain T, Neuro-fuzzy model of homocysteine metabolismJ Genet 2017 96(6):919-26.10.1007/s12041-017-0856-x29321350 [Google Scholar] [CrossRef] [PubMed]
[30]. Naushad SM, Reddy CA, Kumaraswami K, Divyya S, Kotamraju S, Gottumukkala SR, Impact of hyperhomocysteinemia on breast cancer initiation and progression: Epigenetic perspectiveCell Biochem Biophys 2014 68(2):397-406.10.1007/s12013-013-9720-723934182 [Google Scholar] [CrossRef] [PubMed]