Various irrigants have been used so far in dentistry such as saline, distilled water, sodium hypochlorite, Chlorhexidine, Ethylene Diamine Tetra Acetic Acid (EDTA), etc. In spite of the use of various combinations of these irrigating solutions, the complete elimination of the microorganisms is not achieved [4]. Thus, efforts should be continued in the pharmaceutical industry to develop novel products for the treatment of resistant organisms as there is only limited number of agents that are effective against biofilms.
Cyclic lipopeptides, beta-lactamase inhibitors, lantibiotics and cationic bisbiguanides have promising antimicrobial efficacy against various gram-positive micro-organisms. Daptomycin is a cyclic lipopeptide antibiotic produced as a minor component of a complex lipopeptide mixture (A21978C) by the soil actinomycete Streptomyces roseosporus with potent bactericidal activity against most gram-positive organisms including Methicillin Resistant Staphylococcus aureus (MRSA), Vancomycin Resistant Staphylococcus aureus (VRSA) and Enterococci (VRE). It acts by insertion of the lipophilic Daptomycin tail into the bacterial cell membrane, causing rapid membrane depolarisation and a potassium ion efflux, followed by arrest of Deoxyribonucleic Acid (DNA), Ribonucleic Acid (RNA) and protein synthesis resulting in bacterial cell death [5].
Daptomycin along with ceftaroline as the initial combination therapy produces rapid and sustained bactericidal activity and prevents Daptomycin resistance. Ceftaroline is a broad spectrum cephalosporin active against gram-positive cocci including MRSA and VRE. Lantibiotics contain the characteristic polycyclic thioether amino acids lanthionine or methyllanthionine, as well as the unsaturated amino acids dehydroalanine and 2-aminoisobutyric acid produced by a large number of gram-positive bacteria.
Chlorhexidine (CHX) is a cationic bisbiguanide used as intracanal medicament and root canal irrigant because of its various properties such as substantivity, hydrophobic and hydrophilic nature and antimicrobial activity against a wide range of microorganisms [7].
The use of the above-suggested antibiotics when used individually as irrigating solutions reduce the occurrence of E. faecalis from the root canals [2, 7]. Until now no published literature is available in which the combination of cyclic lipopeptide antibiotics with beta-lactamase inhibitors (Daptomycin mixed with Ceftaroline) and lantibiotics (Nisin) are evaluated in a single study. Hence the aim of the present study was to evaluate the antimicrobial efficacy of combination of cyclic lipopeptide antibiotics with beta-lactamase inhibitors (Daptomycin mixed with Ceftaroline), lantibiotics (Nisin) and cationic bisbiguanide (Chlorhexidine) against Enterococcus faecalis biofilm.
Materials and Methods
The study protocol was analysed and approved by the Institutional Review Board of SVSIDS/PEDO/4/2016, India. It was an in-vitro study.
Forty-five single rooted primary teeth were collected from January 2017 to July 2018, Department of Pedodontics and Preventive Dentistry, Sri Venkata Sai Institute of Dental Sciences, Mahabubnagar, Telangana, India. Teeth with 2/3rd root intact were included and teeth having internal and external root resorption and canal calcifications were excluded from the study.
Sample Size Calculation
A sample size calculator (Sample Size Determination in Health Studies, World Health Organization https://www.rnoh.nhs.uk/sites/default/files/sample_size_formula_for_a_comparative_study.pdf) was used for sample size calculation as follows. Power at 90% and β at 10%, the sample size turned out to be 15 teeth in each group. Thus, 45 single-rooted teeth were sectioned along the midsagittal plane and each half was used as a sample.
The extracted teeth were made free from debris by cleaning with 5.25% sodium hypochlorite and were stored in 0.1% thymol solution until used. The tooth sections were decoronated with a diamond disk under water coolant and the root length was standardised to 8 mm. Canal debridement was done using rotary files in a sequence starting from S1, S2 and F1. (Dentsply, India Pvt., Ltd., India) In between change of the files, the canals were irrigated with 5 mL of 3% sodium hypochlorite and 10 mL of saline alternatively. Smoothening was done using the Arkansans stone followed by autoclaving. (Unique clave, C-279, Confident Dental equipments Ltd., India).
E. faecalis suspension was prepared by a growing pure culture of E. faecalis (ATCC 29212) in Mueller Hinton broth and the suspension was standardised in MC FARLAND units. The standardised E. faecalis suspension along with the tooth samples in a test tube was incubated in an incubator at 37°C for three weeks to allow the formation of biofilm. The culture medium was replaced every alternate day to avoid nutrient depletion and accumulation of toxic end products. The bacterial samples incubated in brain heart infusion agar were taken every week to check for cell viability and purity of the culture [8].
After three weeks the tooth samples were transferred to another test tube containing 0.9% 1.5 mL of saline and the biofilm formed was removed from the tooth by vortexing in a cyclo mixer (CM 101, Remi Equipments) and a bacterial emulsion was prepared.
The petridishes were placed in an incubator at 37°C for about 10 minutes to allow the complete absorption of the bacterial emulsion thereby preventing excessive diffusion of the medicaments.
0.001 gm (1 mg) of daptomycin (Glenmark Pharmaceuticals, Mumbai, India) along with ceftaroline (Wuhan Paruisi Trading Co. Ltd, Wuhan, China) was dissolved in 250 μL of distilled water so that each 10 μL of the solution contained 40 μg of daptomycin mixed with ceftaroline. 0.1 g (100 mg) of Nisin (Bimal Pharmaceuticals, Mumbai, India) was dissolved in 100 mL of distilled water so that each 1 μL of the solution contained 1 mg of Nisin.
A pilot study was done using four serial dilutions of the medicaments, daptomycin mixed with ceftaroline and nisin. The various concentrations used were Daptomycin mixed with Ceftaroline at 40 μg, 80 μg, 120 μg, 160 μg and Nisin at 10 mg, 25 mg, 50 mg and 100 mg. Taking into consideration the zone of inhibition, 40 μg and 80 μg of daptomycin mixed with ceftaroline and 25 mg and 50 mg of nisin were taken as the baseline concentrations. The lower concentrations than the baseline concentrations did not exhibit the complete elimination of the microorganism around its surroundings and when higher concentrations were evaluated there was incomplete absorption of the medicaments onto the disk and an overestimation of the antimicrobial efficacy of the medicament was observed. Hence, 40 μg and 80 μg of daptomycin mixed with ceftaroline and 25 mg and 50 mg of nisin were taken. A 20 μL of 2% Chlorhexidine (Stedman Pharmaceuticals Pvt., Ltd., Tamil Nadu) and 3% sodium hypochlorite (Vishal Dentocare Pvt., Ltd., India) were used.
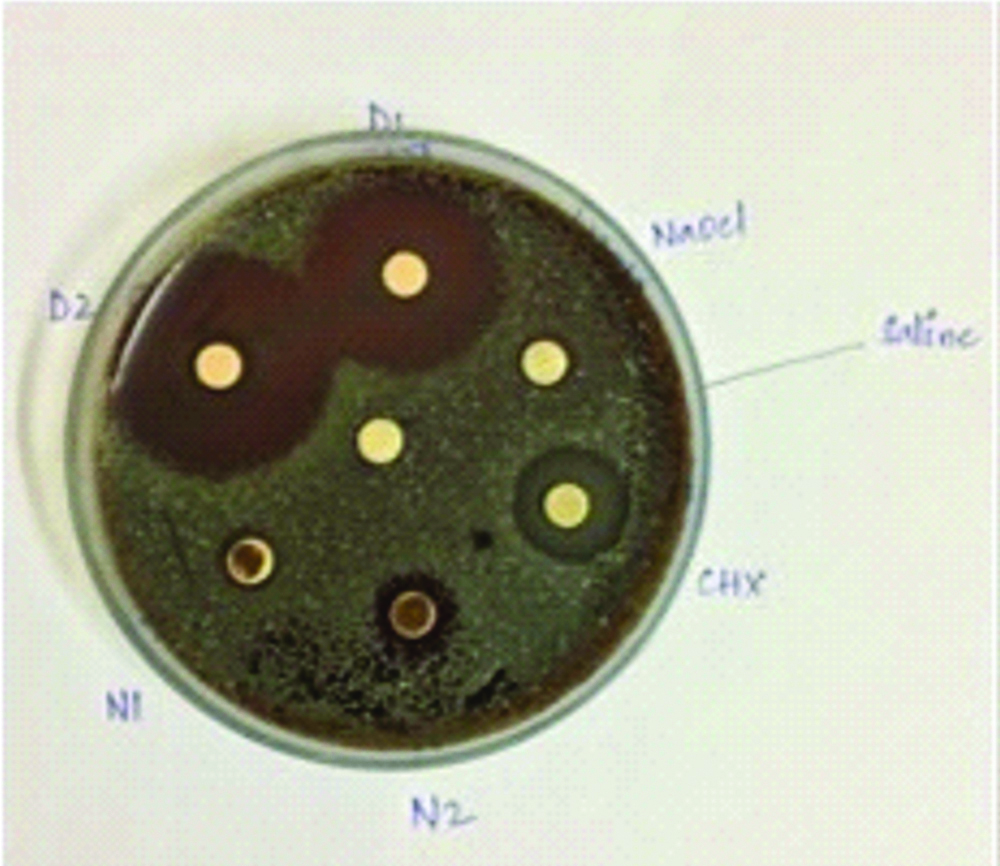
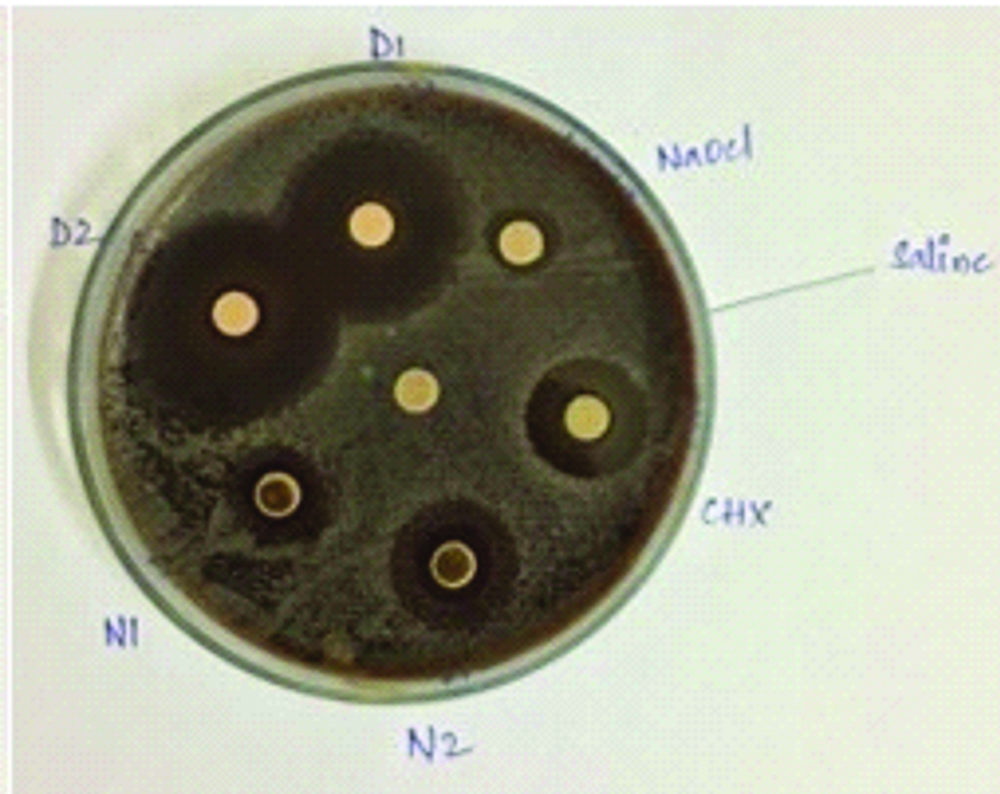
Hi-media disks were placed in the agar petri dishes and the medicament solutions were added to the disks with the help of micropipettes and labelling of the disks were done as D1 (Daptomycin+ceftaroline at 40 μg), D2 (Daptomycin+ceftaroline at 80 μg), N1 (Nisin at 25 mg), N2 (Nisin at 50 mg), CHX (2% Chlorhexidine) and NaOCl (3% sodium hypochlorite). The petridishes were incubated at 37°C and the zones of inhibition were evaluated on 2nd day and 7th day [Table/Fig-1] [8].
Samples with medicament discs.
Statistical Analysis
Data obtained were statistically analysed by One-way Analysis of Variance test and Post-Hoc multiple comparisons test and paired t-test. p-values <0.05 were considered as statistically significant.
Results
Daptomycin mixed with ceftaroline at 40 μg concentration showed minimum zone of inhibition of 11 mm and 11.8 mm, Maximum zone of inhibition of 20.7 mm and 20 mm at 2nd day and 7th day.
Daptomycin mixed with ceftaroline at 80 μg concentration showed highest inhibition of the bacteria with a minimum zone of inhibition of 14.9 mm and 13.8 mm, maximum zone of inhibition of 24 mm and 23.3 mm at 2nd day and 7th day.
Nisin at 25 mg showed a minimum zone of inhibition of 10.4 mm and 10.8 mm, maximum zone of inhibition of 17.3 and 15.7 at 2nd day and 7th day.
Nisin at 50 mg showed a minimum zone of inhibition of 11.8 mm and 12.4 mm, maximum zone of inhibition of 14.9 mm and 14.3 mm at 2nd day and 7th day.
CHX group showed a minimum zone of inhibition of 13 mm and 12.4 mm, maximum zone of inhibition at 14.7 mm and 14.9 mm at 2nd day and 7th day.
NaOCl group showed a minimum zone of inhibition of 6.8 mm and 7.1 mm and the maximum zone of inhibition at 8.9 mm and 9.4 mm at 2nd day and 7th day [Table/Fig-2,3].
Comparison of Zone of inhibition at 2nd day.
Comparison of Zone of inhibition at 7th day.
Intragroup comparison showed no statistical significance when the zone of inhibition was observed after 2nd day and 7th day in all the groups except for NaOCl group (p-value=0.023). The [Table/Fig-4] when intergroup comparison was done, daptomycin mixed with ceftaroline at 80 μg showed highest inhibition of bacteria followed by daptomycin mixed with ceftaroline at 40 μg, Nisin at 50 mg, 2% chlorhexidine, Nisin at 25 mg and 3% NaOCl after 2 days after 2nd day.
Shows comparison of the mean zone of inhibition of all groups at 2nd day and 7th day.
| Groups | Days | n | Min | Max | Mean | S.D | p-value |
|---|
| D1 | 2 | 15 | 11 | 20.7 | 15.6 | 2.9 | 0.212 |
| 7 | 15 | 11.8 | 20 | 14.8 | 2.6 |
| D2 | 2 | 15 | 14.9 | 24 | 18.4 | 3 | 0.072 |
| 7 | 15 | 13.8 | 23.3 | 17.7 | 3.1 |
| N1 | 2 | 15 | 10.4 | 17.3 | 12.7 | 2.2 | 0.65 |
| 7 | 15 | 10.8 | 15.7 | 12.4 | 1.2 |
| N2 | 2 | 15 | 11.8 | 20 | 14.9 | 2.5 | 0.389 |
| 7 | 15 | 12.4 | 18.2 | 14.3 | 1.5 |
| CHX | 2 | 15 | 13 | 17.4 | 14.7 | 1.4 | 0.513 |
| 7 | 15 | 12.4 | 18 | 14.9 | 1.9 |
| NaOCl | 2 | 15 | 6.8 | 10.5 | 8.9 | 1.3 | 0.023 |
| 7 | 15 | 7.1 | 10.7 | 9.4 | 1.4 |
p-value <0.05 was considered as statistical significant
D1-Daptomycin and Ceftaroline at 40 ug, D2-Daptomycin and Ceftaroline at 80 ug, N1-Nisin at 25 mg, N2-Nisin at 50 mg, CHX-2% Chlorhexidine, NaOCl-3% Sodium hypochlorite, Min-Minimum, Max-Maximum, SD-Standard deviation
After seven days daptomycin mixed with ceftaroline at 80 μg had highest zone of inhibition followed by 2% chlorhexidine, daptomycin mixed with ceftaroline at 40 μg, Nisin at 50 mg, Nisin at 25 and 3% NaOCl. Among all the tested groups, daptomycin mixed with ceftaroline at 80 μg showed the highest inhibition of bacteria. The antimicrobial efficacy of nisin was comparable with chlorhexidine.
Discussion
NaOCl group showed the least antimicrobial effect when compared with all the other groups except saline group. The least antimicrobial property of NaOCl might be attributed to various factors such as the time in contact with the target site, concentration at the target site, temperature of the solution and the volume of the solution. Statistical significance was observed when NaOCl was used for two days and seven days (p-value=0.023). There was an increase in the antimicrobial effect after seven days when compared to two days due to longer period of contact of NaOCl with the target site. This is in accordance with the study done by Du T et al., who stated that the killing of bacteria in infected dentin by disinfecting solutions is time-dependent [9]. NaOCl showed a zone of inhibition of 24.35 mm in a study done by Ramezanali F et al., which is much higher than the zone of inhibition in the present study. It might be because of the higher volume of NaOCl i.e., 3 mL used [10].
Apart from NaOCl the other irrigating agent that has a higher bactericidal effect against E. faecalis is 2% (CHX) [11]. CHX found in mouthwash in 0.12% concentration takes too long to kill bacteria during the endodontic procedure and it is not practically possible to leave 0.12% CHX in the canal for several hours [12]. CHX due to its substantivity effect remains antibacterial for several weeks after it is placed in the canal [13,14]. CHX group showed a mean zone of inhibition of 14.7 at 2 days and 14.9 at 7 days which is not statistically significant. (p-value=0.513) The mean zone of inhibition was 17±2 mm and 16.0850 mm in studies conducted by Mistry KS et al., and Palaniswamy U et al., [15,16].
The antimicrobial action of CHX was comparable to that of Nisin at 50 mg concentration after 2 days and 7 days. The antimicrobial action of CHX was higher than that of 3% NaOCl after 2 days and 7 days (p-value ≤0.0001) and lower than that of daptomycin and ceftaroline at 40 μg and 80 μg. This might be due to the different mechanism of action of the medicaments as mentioned earlier. Evidence from the medical field, however, suggests an increase in CHX-resistant bacterial strains. The acquisition of resistance is not related to change in the cell surface hydrophobicity but it is due to alterations in the protein composition [17]. Daptomycin along with ceftaroline as the initial combination therapy produces rapid and sustained bactericidal activity and prevents Daptomycin resistance [18]. Hence a combination of daptomycin mixed with ceftaroline was used in this study.
The antimicrobial action of daptomycin mixed with ceftaroline at 80 μg was higher than daptomycin and ceftaroline at 40 μg. It might be because of the higher concentration of the medicament at the target site. Nisin binds to lipid ll, a cell wall precursor lipid component of target bacteria and disrupts cell wall production [2].
The antimicrobial action of nisin at 50 mg was comparable with that of 2% chlorhexidine and higher than that of 3% sodium hypochlorite. This is in accordance with the study conducted by Somanath G et al., who compared the efficacy of 2% CHX, Linezolid, and Nisin in reducing the Colony Forming Unit (CFU) of E. faecalis at 24 hours, 72 hours, and 1-week intervals and concluded that Nisin was found to be most effective in reducing the bacterial count of Enterococcus faecalis in one week and its action was found to be comparable with Chlorhexidine [2].
Turner SR et al., stated that Nisin is comparable with calcium hydroxide in its ability to eliminate E. faecalis both within the root canal and associated canal wall radicular dentine in vitro [19]. According to Balto HA et al., MTAD, when used in combination with Nisin, appears to be effective and potent as intracanal irrigating solution [20]. According to Tong Z et al., Nisin might be a suitable and effective drug in the prevention of dental caries and root canal therapies [21]. The antimicrobial action of nisin was lower than that of daptomycin and ceftaroline, it might be because of the low solubility of nisin which was observed during the present study.
Limitation
The study is an in-vitro study the effect on the underlying tooth buds is not known. The biocompatibility of the medicaments to the oral environment could not be assessed.
Conclusion
However, the limitation of its usage is because of the cost and availability. Daptomycin mixed with ceftaroline at 80 μg showed better antibacterial effect than all the tested groups but the cost and availability make them difficult to use as an intracanal irrigant.
Fututre Recommendation: The natural ingredient Nisin was found to have a similar antimicrobial activity to that of chlorhexidine and can be used as a substitute.
p-value <0.05 was considered as statistical significant
D1-Daptomycin and Ceftaroline at 40 ug, D2-Daptomycin and Ceftaroline at 80 ug, N1-Nisin at 25 mg, N2-Nisin at 50 mg, CHX-2% Chlorhexidine, NaOCl-3% Sodium hypochlorite, Min-Minimum, Max-Maximum, SD-Standard deviation