Introduction
Retinopathy Of Prematurity (ROP) is one of the leading causes of preventable blindness in our country. The minor risk factors responsible for development of such at Neonatal Intensive Care Unit (NICU) is still under discovery. It need multiple trials to conclude or have guidelines on when to screen an infant. In order to make an attempt to fill the above knowledge gap, this study was conducted at our rural tertiary centre.
Aim
To determine the need for early screening for ROP before discharge. Also, to study the relation between postnatal weight gain and risk of developing ROP and to determine the usefulness of CHOP-ROP (Children’s Hospital of Philadelphia-ROP) model in Indian population in order to use it as a predictor model for ROP screening purpose.
Materials and Methods
This was a retrospective two year study of infants admitted in NICU between June 2016-May 2018 at Adichunchanagiri Institute of Medical Sciences, a rural tertiary care centre. ROP screening was carried out as per National guidelines and objective of the study. A cohort of 75, was eligible for the study; in these infants ROP screening was done before discharge, looked for postnatal weight gain and its relation to development of ROP. Also the CHOP – ROP algorithm was used to classify those who needed and not needed the early screening.
Results
Of 75 infants studied, all infants were screened for ROP before discharge, out of which 60 (80%) were not found to have ROP and 15 (20%) were found to have ROP, no screened out infant developed ROP successively. Among the 15 (20%) infants who developed ROP, 11 (73.3%) infants were having an average weight gain less than 10 gm/day and we found a positive correlation with development of ROP (p=0.101), Fischer-Exact test. By using CHOP-ROP algorithm it was found that, two infants were classified eligible for screening out of 75 infants and both infants had ROP; remaining 73 (97.3%) were classified as not eligible for screening and 13 (86.7%) of these developed ROP (p=0.003, Fischer-Exact test).
Conclusion
Early screening of infants for ROP has been as effective as the national guidelines and in fact has helped in early identification of cases. Also, we found that poor postnatal weight gain has significant correlation with development of ROP. CHOP-ROP model although is statistically significant but has failed to classify large number of cases as risk group for ROP.
Introduction
Retinopathy Of Prematurity (ROP) is one of the leading causes of preventable blindness in children, especially in India [1]. Currently middle income countries like India and others are said to be suffering from “third epidemic” of blindness from ROP [2-4]. ROP screening is being followed in our country as per neonatology forum guidelines, which include: (i) all infants less than 1750 gm and/or Gestational Age (GA) <34 weeks; (ii) infants between 34 to 366/7 weeks gestation or birth weight between 1750 and 2000gm should be screened for risk factors for developing ROP. The risk factors for developing ROP are mechanical ventilation, prolonged oxygen support and haemodynamic instability [5].
ROP develops in two phases, a hypoxic mechanical phase and a subsequent proliferative clinical phase. These phases result from alteration in serum Insulin-like Growth Factor-1 (IGF-1), which is a somatic growth factor; retinal Vascular Endothelial Growth Factor (VEGF), a hypoxia induced vasoproliferative factor necessary for normal retinal vascular development [6]. IGF-1 plays a permissive role in VEGF mediated vessel growth during development [7,8]. Loss of maternal IGF-1 and loss of endogenous IGF-1 production in premature infants lead to poor retinal vessel growth, retinal hypoxia and the development of neovascular ROP [9]. Slow post-natal weight gain is a surrogate measure for low serum IGF-1 and has been found to be an excellent predictor for the subsequent development of severe ROP [10]. The slow weight gain is a surrogate measure for a slow rise in serum IGF-1, which result in insufficient activation of retinal VEGF by IGF-1 and poor retinal vascular growth early in post natal life [11,12]. Hence there is a growing need to test this correlation between post natal weight gain and onset of ROP.
In our country, all the infants falling in eligible criteria as per national guidelines are subjected to ROP screening. These examinations are physically stressful for infants and labor intensive for doctors. Besides these examination yield fewer than 10% of ROP cases, infact in the multipariate study conducted at US, Canadian and UK shows less than 5% of infants needed treatment by laser surgery [13-15].
There are various models that have been proposed in order to reduce the number infants requiring examinations. Multiple growth based models have been developed including WINROP (Weight, Insulin like growth factor-1, neonatal, ROP), CHOP-ROP score and CO-ROP (Colorado ROP). However, there are limited studies in India which has determined the usefulness of these models.
In a developing country like India, it is important to have early detection of ROP and so we have screened all the infants fitting in eligibility criteria and identified the infants with ROP. The recommendation for first ROP screening by AAP (American Academy of Pediatrics) guidelines suggest that infants less than 26 weeks are examined at postnatal age of 6-8 weeks, those born at 27-28 weeks at the postnatal age of 5 weeks, those born at 29-30 weeks at postnatal age of 4 weeks those born after 30 weeks at postnatal age of 3 weeks [16].
According to UK ROP guidelines, babies born before 27 weeks GA the first ROP screening examination should be undertaken at 30 to 31 weeks postmenstrual age; Babies born between 27 and 32 weeks GA the first ROP screening examination should be undertaken between 4 to 5 weeks postnatal age; Babies >32 weeks gestational age but with birthweight <1501 gm, the first ROP screening examination should be undertaken between 4-5 weeks of postnatal age [17].
In India, the first screening is recommended between 2-3 weeks for infants born before 28 weeks gestational age or with the a birth weight less than 1200 gm and for infants born between GA of 28-34 weeks not later than 30 days or 4 weeks [18].
In our study, we have looked for the correlation between postnatal weight gain and development of ROP especially in the Indian rural population. Also, we sought to evaluate the usefulness of CHOP-ROP algorithm in Indian population, if it could successfully predict the infant at risk. As a secondary objective, we have looked for the outcome by modifying the ROP guidelines to initiate the first screening before discharge, irrespective of post menstrual age.
Materials and Methods
A retrospective observational study was done on all the eligible infants admitted in NICU between June 2016 to May 2018. Approval for the study was taken from institute ethic committee (AIMS/IEC/1568/2018). The infant’s parents were explained in detail and written consent was taken to make their infant as study group.
Inclusion criteria: Babies less than or equal to 34 weeks and/or less than or equal to 1750 gm and those infants between 1750 gm to 2000 gm with risk factors like mechanical ventilation, prolonged oxygen support, hypotension. However for the application of CHOP-ROP algorithm infants less than 34 weeks only were considered irrespective of birth weight.
Exclusion criteria: Babies who had non physiological weight gain like cardiac failure, hydrocephalus, excess weight due to syndrome, infants gone against medical advice were excluded from the study.
Methods
Data were retrospectively collected from medical records of infants that included birth weight, GA and weight measurements, gender, weight gain and other complications during NICU stay. Average weight gain was calculated by dividing the total weight gain in one week by 7; if infant was at NICU more than a week, then average of weekly average was take. Such average weight gain was looked in all babies till their date of ROP screening.
All the eligible infants were screened for ROP if had developed the same, irrespective of staging. The infants were further categorised into daily average weight gain of less than 10 gm; 20-30 and more than 30 gm, and accordingly the number of ROP cases in each group was listed.
For the same cohort, CHOP-ROP algorithm was applied [Table/Fig-1], assessed for how many infants were falling above the threshold of 0.0140 and classified as eligible for ROP screening and those infants were looked for having actually developed ROP. Thus the algorithm was tested in all infants less than 34 weeks.
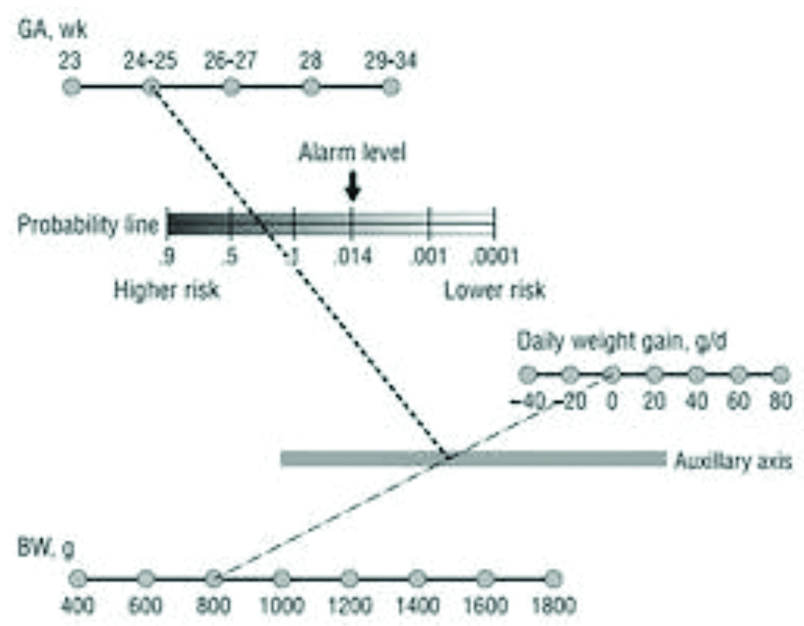
Further, eligible infants as per national guidelines were screened before discharge and those babies who were asked to follow-up was successively looked for if any new development of ROP. Thus, the outcome of early screening was noted.
Statistical Analysis
Descriptive and inferential statistical analysis has been carried out. The results in our study which are on continuous measurements are presented on Mean±SD and results on categorical measurements are presented in percentage (%), level of significance was taken as 5%.
The Chi-square/Fisher-Exact test has been used to find the significance of study parameters on categorical scale between two or more groups in our study, Non-parametric setting for Qualitative data analysis. We used Fisher-Exact test used when cell samples are very small. Suggestive significance was p-value less than 0.05.
Results
Of the total 75 infants, there were 34 females (45.3%) . Majority of the infants (40,53.3%) were between 33-36 weeks [Table/Fig-2]. The data on the birth weight is depicted in [Table/Fig-3].
Period Of Gestation (POG) of our study group by Last Menstrual Period/Expected Date of Delivery.
| POG in weeks | No. of patients |
|---|
| 29-32 | 30 (40%) |
| 33-36 | 40 (53.3.%) |
| 37-40 | 5 (6.7%) |
| Total | 75 (100%) |
Distribution of birth weight of the study group.
| Birth weight (kg) | No. of patients (n=75) |
|---|
| <1 | 7 (9.3%) |
| 1-1.5 | 30 (40%) |
| 1.5-2 | 23 (30.7%) |
| 2-2.5 | 15 (20%) |
| 2.5-4 | 0 |
| >4 | 0 |
Of the above cohort, 7 infants (9.3%) were less than 1 kg, 30 infants (40%) were between 1-1.5 kg, 23 infants (30.7%) were between 1.5 to 2 kg, 15 infants (20%) were between 2 to 2.5 kg and none of the babies weighed more than 2.5 kg [Table/Fig-3].
Among the total number of infants screened, 60 infants (80%) had no ROP and only 15 (20%) had developed ROP. In the above 60 infants without ROP, 31 (51.7%) were females, 29 (48.3%) were males, and in those 15 infants with ROP 3 (20%) were females and 12 (80%) were males.
[Table/Fig-4] shows the distribution of the ROP screening outcome with respect to GA. Amongst the total 30 infants with GA 29-32 weeks, 12 infants developed ROP. In group of 33-36 weeks of POG 3 infants developed ROP whereas amongst 37-40 weeks GA infants no infant developed ROP.
Period Of Gestation (POG) in week’s distribution with the incidence of ROP of patients studied.
| POG in weeks | ROP | Total |
|---|
| No | Yes |
|---|
| 29-32 | 18 (30%) | 12 (80%) | 30 (40%) |
| 33-36 | 37 (61.7%) | 3 (20%) | 40 (53.3%) |
| 37-40 | 5 (8.3%) | 0 (0%) | 5 (6.7%) |
| Total | 60 (100%) | 15 (100%) | 75 (100%) |
p=0.002**; Significant; Fisher-Exact Test
[Table/Fig-5] shows that among the total number of infants screened, infants less than 1 kg without ROP were 5 (8.3%) with ROP were 2 (13.3%); of the 30 infants between 1 to 1.5 kg 17 (28.3%) had no ROP, 13 (86.7%) had ROP. This group constituted the highest percentage of infants with ROP 86.7%. Amongst the 23 infants between 1.5 to 2 kg, none of them developed ROP and so was infants between 2-2.5 kg, 2.5 to 4 kg and more than 4 kg. We can infer that most statistically significant group with ROP was 1-1.5 kg. Since the total number of infants under 1 kg were numerically less, statistical significance could not be established.
Birth weight (kg) distribution with the incidence of ROP of patients studied.
| Birth weight (kg) | ROP | Total (n=75) | p-value |
|---|
| No (n=60) | Yes (n=15) |
|---|
| <1 kg | 5 (8.3%) | 2 (13.3%) | 7 (9.3%) | 0.622 |
| 1-1.5 | 17 (28.3%) | 13 (86.7%) | 30 (40%) | <0.001** |
| 1.5-2 | 23 (38.3%) | 0 (0%) | 23 (30.7%) | 0.004** |
| 2-2.5 | 15 (25%) | 0 (0%) | 15 (20%) | 0.030* |
| 2.5-4 | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| >4 | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| 100% | 100% | 100% | |
Chi-Square/Fisher Exact Test
From above [Table/Fig-6] we can infer that, amongst the 32 (42.6%) infants with average weight gain less than 10 gm/day, 10 (66.6%) developed ROP, 22 (36.6%) did not develop ROP. In 13 (17.3%) infants between daily average weight gain 10-20 gm, 3(20%) developed ROP where as 10 (16.6%) did not get ROP. Amongst 20-30 gm average weight gain daily 25 (33.3%) of infants were there in which 2 (13.3%) developed ROP whereas 23 (38.3%) did not develop ROP. Amongst more than 30 gm average daily weight gain 5 (6%) none of them developed ROP.
Average weight gain and its correlation with development of ROP.
| Weight (gm/day) | Total | ROP |
|---|
| | Yes | No |
|---|
| <10 | 32 (42.6%) | 10 (66.6%) | 22 (36.6%) |
| 10-20 | 13 (17.3%) | 3 (20%) | 10 (16.6%) |
| 20-30 | 25 (33.3%) | 2 (13.3%) | 23 (38.3%) |
| >30 | 5 (6%) | 0 | 5 (8.3%) |
| Total | 75 (100%) | 15 (100%) | 60 (100%) |
Pearson chi-square= 6.108; p-value= 0.106464
We can thus conclude that maximum number of infants with ROP had less than 10 gm/day weight gain; thus lesser the weight gain more the chances of ROP.
We can infer from [Table/Fig-7] that as per CHOP-ROP eligibility criteria 2 (3.1%) of them qualified for screening and both of them had developed ROP but 63 (96.9%) did not qualify the screening criteria, 13 (86.6%) of them yet developed ROP. Although this table is statistically significant, the CHOP-ROP algorithm has missed out a lot of ROP cases.
The correlation between those have been eligible to be screened under CHOP ROP model with actual number of cases which had developed ROP.
| ROP | |
|---|
| Classified for screening eligibility by CHOP ROP model | No | Yes | |
|---|
| No | 50 (100%) | 13 (86.6%) | 63 (96.9%) |
| Yes | 0 (0%) | 2 (13.4%) | 2 (3.1%) |
| Total | 50 (100%) | 15 (100%) | 65 (100%) |
Pearson chi-square=8.417; p-value=0.003718
Discussion
With respect to post natal weight gain and development of ROP, there are studies which are done in Asian population Vinekar A et al., study of regaining birth weight and predicting ROP showed those infants who regained birth weight faster had least risk of developing ROP unlike those who gained weight slower [19]. Also, the study done by Binenbaum G on algorithms for predictors of ROP says slow postnatal growth is a surrogate measure for low IGF-1, which is an important risk factor for severe ROP [20].
However the study done by Biniwale M et al., demonstrates that faster weight gain and higher IGF-1 have positive correlation with development of ROP. Also, various studies like WIN_ROP, G-ROP, CHOP-ROP have demonstrated that post natal weight gain can act as a surrogate marker of IGF-1 and postnatal weight gain velocity is proportional to development of ROP [21].
In our study, we have determined that infants with weight gain velocity <10 gm per week have potential to develop ROP (p-value 0.061) though not statistically significant. Hence, our study suggests that post natal weight gain velocity has an inverse relation to development of ROP, these infants fall in higher risk for ROP and these infants need to be screened earlier than non-risk infants in order to avoid ROP burden to the society.
Further there are various algorithms developed in order to identify ‘at risk’ infants and earlier screening like WIN-ROP, PINT-ROP, G-ROP scores, CHOP-ROP. We have adopted CHOP-ROP model and looked for its application in Indian rural population. The study done by Binenbaum G et al., on CHOP post natal weight gain, birth weight and GA risk model showed that CHOP-ROP model had sensitivity of 98% but specificity of only 53%, PPV was 17% and NPV was 100% [22]. Likewise another study done by Binenbaum G et al., on validation of CHOP-ROP model demonstrated that if cut-off point 0.0140 was used then it showed sensitivity of 98.5% and specificity of 36.4% [23].
In our study, although we have achieved statistical significance with p-value but the CHOP-ROP algorithm has missed significant number of infants who had developed ROP. Since ours was a retrospective study, we did not solely depend on the algorithm for prediction, consequently we did not miss any infant in the screening of ROP. In a middle income country like India, it is highly important to identify the morbidity at the earliest, as the initial stage of any disease will have lesser financial burden. The conventional 30 day screening of ROP in eligible infants has yielded increased number of infants with advanced ROP including AP-ROP. Besides these infants require multiple sittings of treatment which increase the financial burden of ROP. In our tertiary rural center, where most of the parents depend on the daily wages and it is hard to keep the infants in hospital for the purpose of only ROP screening though infant is clinically stable. The adoption of early screening i.e screening before discharge reduces inconvenience to the parents and those babies who are indefinite of diagnosis may be asked to follow-up. In a study done by Vinekar A et al., it is shown that initiating screening before ROP discharge concluded that early enrolment of infants for ROP screening ensure superior yield compared to the conventional screening [24]. Like wise we have successfully demonstrated that early screening has not missed a single case of ROP. This outcome suggest the need for modifying the screening guidelines in India.
Limitation
The study which we have conducted is a retrospective study hence has its own fallacies. In order to prove CHOP-ROP model we need multi-centre trial since such studies have not been much conducted in our country.
Conclusion
Postnatal weight gain can act as a surrogate marker for the development of ROP and hold an inverse relationship with the postnatal weight gain velocity. Also, CHOP-ROP model application is limited in our study but needs more studies to validate its usage in Asian population. Early screening for ROP has equal prospect of identifying ROP and can reduce advanced ROP as well as financial burden on parents.
p=0.002**; Significant; Fisher-Exact Test
Chi-Square/Fisher Exact Test
Pearson chi-square= 6.108; p-value= 0.106464
Pearson chi-square=8.417; p-value=0.003718
[1]. Gilbert C, Rahi J, Eckstein M, O’Sullivan J, Foster A, Retinopathy of prematurity in middle-income countriesLancet 1997 350:12-14.10.1016/S0140-6736(97)01107-0 [Google Scholar] [CrossRef]
[2]. Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: Implications for screening programsPediatrics 2005 115:e518-25.10.1542/peds.2004-118015805336 [Google Scholar] [CrossRef] [PubMed]
[3]. The Global Action Report on Preterm Birth. United Nations 2012. Born too soon. Available from: http://www.who.int/pmnch/media/news/2012/201204_borntoosoonreport.pdf. Accessed June 15, 2015 [Google Scholar]
[4]. Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C, Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010Pediatr Res 2013 74(Suppl 1):35-49.10.1038/pr.2013.20524366462 [Google Scholar] [CrossRef] [PubMed]
[5]. Pejaver RK, Vinekar A Bilagi A. National Neonatology Forum’s Evidence Based Clinical Practice Guidelines 2010. Retinopathy of Prematurity (NNF India, Guidelines) [cited 2015 Jun 29]; Available from: http://www.ontop-in.org/ontop-pen/Week-12-13/ROP NNF Guidelines.pdf. Accessed June 15, 2015 [Google Scholar]
[6]. Pierce EA, Foley ED, Smith LE, Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurityArch Ophthalmol 1996 114(10):1219-28.10.1001/archopht.1996.011001404190098859081 [Google Scholar] [CrossRef] [PubMed]
[7]. Binenbaum G, Ying GS, Quinn GE, Dreiseitl S, Karp K, Roberts RS, A clinical prediction model to stratify ROP risk using postnatal weight gainPediatrics 2011 127(3):e607-14.10.1542/peds.2010.224010.1542/peds.2010-224021321036 [Google Scholar] [CrossRef] [CrossRef] [PubMed]
[8]. Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurityProc Natl Acad Sci USA 2001 98:5804-08.10.1073/pnas.10111399811331770 [Google Scholar] [CrossRef] [PubMed]
[9]. Wu C, Vanderveen DK, Hellström A, Löfqvist C, Smith LE, Longitudinal postnatal weight measurements for the prediction of retinopathy of prematurityArch Ophthalmol 2010 128(4):443-47.10.1001/archophthalmol.2010.3120385939 [Google Scholar] [CrossRef] [PubMed]
[10]. Hellström A, Engström E, Hård AL, Albertsson-Wikland K, Carlsson B, Niklasson A, Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birthPediatrics 2003 112(5):1016-20.10.1542/peds.112.5.101614595040 [Google Scholar] [CrossRef] [PubMed]
[11]. Löfqvist C, Andersson E, Sigurdsson J, Engström E, Hård AL, Niklasson A, Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurityArch Ophthalmol 2006 124(12):1711-18.10.1001/archopht.124.12.171117159030 [Google Scholar] [CrossRef] [PubMed]
[12]. Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptorNat Med 1999 5(12):1390-95.10.1038/7096310581081 [Google Scholar] [CrossRef] [PubMed]
[13]. Early Treatment for Retinopathy of Prematurity Cooperative GroupRevised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trialArch Ophthalmol 2003 121:1684-94.10.1001/archopht.121.12.168414662586 [Google Scholar] [CrossRef] [PubMed]
[14]. Chiang MF, Arons RR, Flynn JT, Starren JB, Incidence of retinopathy of prematurity from 1996 to 2000: Analysis of a comprehensive New York state patient databaseOphthalmology 2004 111(7):1317-25.10.1016/j.ophtha.2003.10.03015234131 [Google Scholar] [CrossRef] [PubMed]
[15]. Lee SK, Normand C, McMillan D, Ohlsson A, Vincer M, Lyons C, Evidence for changing guidelines for routine screening for retinopathy of prematurityArch Pediatr Adolesc Med 2001 155(3):387-95.10.1001/archpedi.155.3.38711231807 [Google Scholar] [CrossRef] [PubMed]
[16]. Fierson WM, American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening Examination of Premature Infants for Retinopathy of PrematurityPediatrics 2013 131:189-95.10.1542/peds.2012-299623277315 [Google Scholar] [CrossRef] [PubMed]
[17]. [Internet]. Rcophth.ac.uk. 2019 [cited 21 March 2019]. Available from: https://www.rcophth.ac.uk/wp-content/uploads/2014/12/2008-SCI-021-Guidelines-Retinopathy-of-Prematurity.pdf [Google Scholar]
[18]. Pejaver RK, Vinekar A, Bilagi A. National Neonatology Forum’s Evidence Based Clinical Practice Guidelines 2010. Retinopathy of Prematurity (NNF India, Guidelines) [cited 2015 Jun 29]; Available from: http://www.ontop-in.org/ontop-pen/Week-12-13/ROP NNFGuidelines.pdf. [Accessed June 15, 2015] [Google Scholar]
[19]. Vinekar A, Mangalesh S, Mallavarapu M, Jayadev C, Sharma P, Shetty B, Regaining birth weight and predicting ROP-a prospective, pilot studyAnn Eye Sci 2017 2:5010.21037/aes.2017.03.05 [Google Scholar] [CrossRef]
[20]. Binenbaum G, Algorithms for the prediction of retinopathy of prematurity based on postnatal weight gainClin Perinatol 2013 40:261-70.10.1016/j.clp.2013.02.00423719309 [Google Scholar] [CrossRef] [PubMed]
[21]. Biniwale M, Weiner A, Sardesai S, Cayabyab R, Barton L, Ramanathan R, Early postnatal weight gain as a predictor for the development of retinopathy of prematurityJ Matern Fetal Neonatal Med 2017 32(3):429-33.10.1080/14767058.2017.138190228920494 [Google Scholar] [CrossRef] [PubMed]
[22]. Binenbaum G, Ying G, Quinn G, Huang J, Dreiseitl S, Antigua J, The CHOP Postnatal weight gain, birth weight, and gestational age retinopathy of prematurity risk modelArchives of Ophthalmology 2012 130(12):156010.1001/archophthalmol.2012.252423229697 [Google Scholar] [CrossRef] [PubMed]
[23]. Binenbaum G, Ying G, Tomlinson L, Validation of the Children’s Hospital of Philadelphia Retinopathy of Prematurity (CHOP ROP) ModelJAMA Ophthalmology 2017 135(8):87110.1001/jamaophthalmol.2017.229528715553 [Google Scholar] [CrossRef] [PubMed]
[24]. Vinekar A, Jayadev C, Mangalesh S, Kurian M, Dogra M, Bauer N, Initiating retinopathy of prematurity screening before discharge from the neonatal care unit: Effect on enrolment in Rural IndiaJournal of Indian Paediatrics 2016 53(2):S107-11. [Google Scholar]