Introduction
Acute pancreatitis is an inflammatory disease of the pancreas with grade of severity ranging from very mild indolent course to severely fatal necrotising pancreatitis. Early detection of the disease severity and early intervention is crucial for favourable outcome. Several prognostic markers and severity scores have been developed and studied to predict the disease severity in acute pancreatitis; but not a single one is ideal.
Aim
To study Red-Cell Distribution Width (RDW) to Total Platelet Count (TPC) ratio, shortly RPR (Red Cell Distribution Width to Platelet Ratio); as a prognostic indicator in acute pancreatitis.
Materials and Methods
A total of 60 patients with a diagnosis of acute pancreatitis were included in this prospective study. Patients were divided into two groups; mild acute pancreatitis group (MILD AP) and severe acute pancreatitis group (SEVERE AP) according to the revised Atlanta classification 2012 (the severe group includes both moderate and severe acute pancreatitis patients). Patients were followed up till the final outcome that means either death or discharge from the hospital. RPR (RDW/TPC) value was calculated from the complete blood count report for all patients. Various prognostic scores including RANSON’s score, SOFA score, BISAP score, APACHE II, modified Glasgow score were calculated for all patients. RPR and other score values were compared between the discharged and dead patients as well as between the MILD AP group and Severe AP group. Statistical analysis was done using SPSS 21 software and p-value was calculated using the unpaired t-test.
Results
The RPR (RDW/TPC) value was found to be significantly higher in patients with severe acute pancreatitis as well as in patients in whom the final outcome was death (p-value <0.001). Using the ROC curve analysis, it was found that at a cut-off value of 0.045 (RDW-CV in % and TPC in x1000/μL) the RPR has a sensitivity of around 90% and a specificity of around 73% in predicting the severity of the disease and with a cut-off value of 0.071, RPR has a sensitivity of around 82% and a specificity of around 96%, in predicting mortality in acute pancreatitis patients.
Conclusion
From this study we conclude that RPR is a useful marker of disease severity in acute pancreatitis, especially in early stage.
Blood cell parameters, Severe acute pancreatitis, Severity scores, Systemic inflammatory response
Introduction
Acute pancreatitis is one of the most common gastrointestinal admissions and currently it is in an increasing trend [1]. From Indian population no data is available regarding the actual incidence of the disease. Only some idea of incidence can be obtained from patients admitted in different tertiary care centres in India (in SP medical college Bikaner, Rajasthan 50 cases per year, Indira Gandhi medical college Shimla, Himachal Pradesh 123 cases per year) [2,3]. The Incidence of the disease in USA is 49.2 cases/1,00,000 USA populations [4]. In most of the cases the severity of the disease is mild with a favourable outcome (around 70%-85% cases). But in rest 15-30% cases the disease may not have a benign course and a mortality rate of around 30% is reported, even with best supportive care in the setting of very severe disease [5]. The revised Atlanta classification 2012 [6] divides the disease progression into two phases; the early phase which lasts for first two weeks and a late phase of next two weeks. The clinical picture of early phase is dominated by systemic inflammatory response and the resulting multiorgan failure from it, known as the Systemic Inflammatory Response Syndrome (SIRS) phase. The second phase starts after two weeks where the counter inflammatory reactions predominate which lead to septic complications. Mortality in initial two weeks is due to multiorgan dysfunction resulting from SIRS and in the second phase it is mostly due to the infective complications of the localised collections [6,7].
The new revised Atlanta guidelines [6] gave more stress on organ failure to predict outcome in acute pancreatitis patients rather than the local complications as was present in the 1992 Atlanta guidelines [8]. So, SIRS and the resulting organ failure better predicts the outcomes in acute pancreatitis [9]. In SIRS phase due to the effect of inflammatory cytokines, there occurs release of premature erythrocytes into the circulation and a resultant increase in RBC distribution width [10,11]. It has been also found that when the SIRS becomes more severe the total platelet count decreases. Although, the aetiology is not certain, this may be due to the inhibitory effect of inflammatory cytokines on bone marrow or may be due to the Disseminated Intravascular Coagulation (DIC) and the resultant consumption coagulopathy occurring at late stage of sepsis and SIRS [12,13]. So, ratio of RDW to TPC known as RPR can be evaluated as a prognostic index to know the degree of severity of SIRS and hence the outcome in acute pancreatitis.
Materials and Methods
It was a prospective study, conducted on patients with confirmed diagnosis of acute pancreatitis, admitted and treated in SCB Medical College and Hospital, Cuttack, a tertiary care hospital in east India, during the period-August 2014 to July 2016. Ethical clearance was taken from the institutional ethical committee (SCB, IEC 14/201) of the hospital. Consent was also obtained from the patients or their relatives before they were included in the study. As per the IAP guidelines we diagnosed a case as acute pancreatitis when it fulfilled 2 out of 3 of the following criteria: clinical (upper abdominal pain), laboratory (serum amylase or lipase level more than three times upper limit of normal, normal serum amylase 40-140 U/L, normal serum lipase 0-160 U/L) and/or imaging (CT, MRI, and ultrasonography) feature suggestive of acute pancreatitis [14]. Patients with previous known haematological or coagulation disorders were excluded from the study.
In all acute abdomen cases suspicious of acute pancreatitis, a detailed history of the disease was taken and a detailed clinical examination was done after assessment of the vitals of the patient. Management was done according to IAP/APA guidelines for management of acute pancreatitis [14]. All patients were followed up till the final outcome (Death or discharge from the hospital). All the required clinical and laboratory parameters were recorded in a case proforma. Red cell distribution to total platelet count ratio shortly known as RPR was calculated from the complete blood count.
RPR=RDW-CV in percentage/Total platelet count in x1000/μL
RDW-CV is calculated from the red cell distribution width histogram by automated haematology analysers used for complete blood count. The RDW-CV is calculated from the width of the histogram at 1 SD from the mean divided by MCV [15]. Different severity scores like RANSON’s score, MODIFIED GLASGOW score, APACHE II score, BISAP score and SOFA scores were calculated and recorded in a master chart for statistical analysis [16-20]. The new revised Atlanta classification (2012) divides the disease severity in acute pancreatitis into three categories (mild, moderately severe and severe pancreatitis). But for statistical analysis we divided patients into two groups; mild acute pancreatitis group (MILD AP) and severe acute pancreatitis group (SEVERE AP which includes both moderate and severe acute pancreatitis patients).
Statistical Analysis
Statistical analysis was done using SPSS 21 software (IBM inclusive, Chicago) and p-value was calculated using unpaired t-test.
Results
Out of the total 60 patients included in the study, 31 were male and 29 patients were female. Mean age in our patients was 38.86 (range 15 to 72). A total of 49 patients were cured of the disease and discharged and 11 patients died of the disease. The mean value of the different severity scores between the patients who were discharged against patients who were died and the statistical difference between them is given in [Table/Fig-1].
Comparison different scores between patients who were discharged vs. died.
| Factors | In patients who were cured and discharged (mean value) | In patients who were expired (mean value) | p-value (unpaired t-test) |
|---|
| Age (years) | 38.816 | 39.091 | 0.947 |
| APACHE II score | 4.224 | 15.545 | <0.001 |
| RANSON’s score | 1.653 | 5.364 | <0.001 |
| Modified glassgow score | 1.653 | 3.636 | 0.001 |
| SOFA score | 1.694 | 8.636 | <0.001 |
| BISAP score | 0.551 | 3.091 | <0.001 |
| CRP (in mg/L) | 83.531 | 156.273 | 0.001 |
| RPR=RDW/TPC | 0.044 | 0.089 | <0.001 |
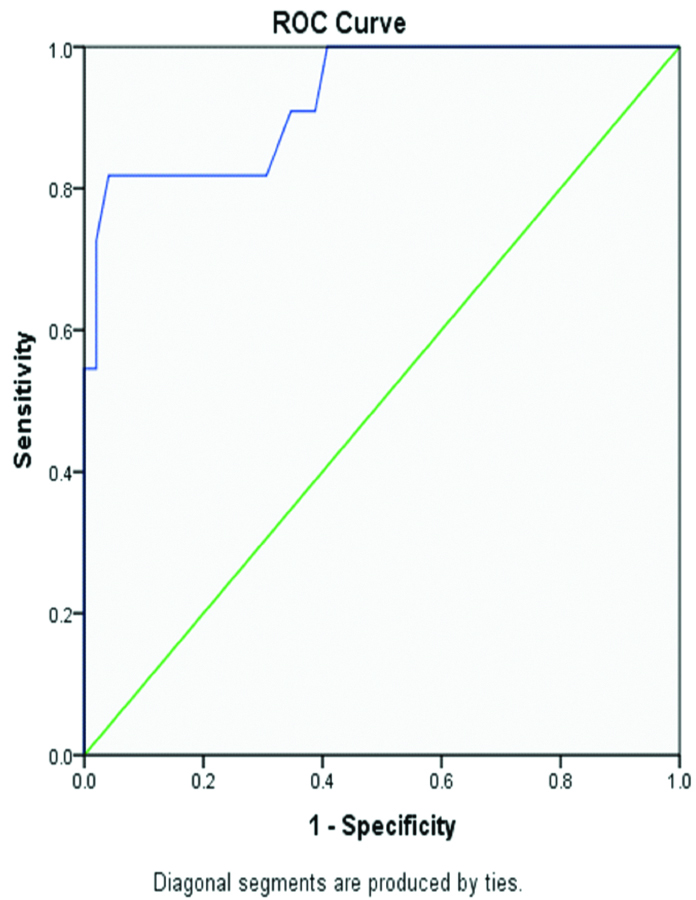
The mean RPR value was 0.044 in the survivor group and 0.089 in the death group, (RDW as RDW-CV% and TPC in x1000/μL). When a Receiver Operating Characteristic (ROC) curve was plotted, it was found that at a cut-off value of 0.071 RPR has a sensitivity of 82% and specificity of 96% in predicting mortality in patients with acute pancreatitis [Table/Fig-2]. According to the revised Atlanta classification 31 patients included in our study were having mild form of acute pancreatitis (MILD AP Group), whereas 29 patients were having moderately severe or severe form of the disease (SEVERE AP Group). The mean RPR value and other score mean values for these two different groups are given in the [Table/Fig-3].
ROC curve of RPR as an indicator of mortality in patients with AP.
Comparison of different scores between patients with mild AP vs. patients with severe AP.
| Factors | In patients with mild acute pancreatitis | In patients with moderate to severe acute pancreatitis | p-value (unpaired t-test) |
|---|
| Age (years) | 36.677 | 41.207 | 0.155 |
| APACHE II score | 2.065 | 10.828 | <0.001 |
| RANSON’s score | 0.774 | 4 | <0.001 |
| Modified glassgow score | 0.839 | 3.276 | <0.001 |
| SOFA score | 0.903 | 5.172 | <0.001 |
| BISAP score | 0.194 | 1.897 | <0.001 |
| CRP (in mg/L) | 56.226 | 140.31 | <0.001 |
| RPR=RDW/TPC | 0.038 | 0.068 | <0.001 |
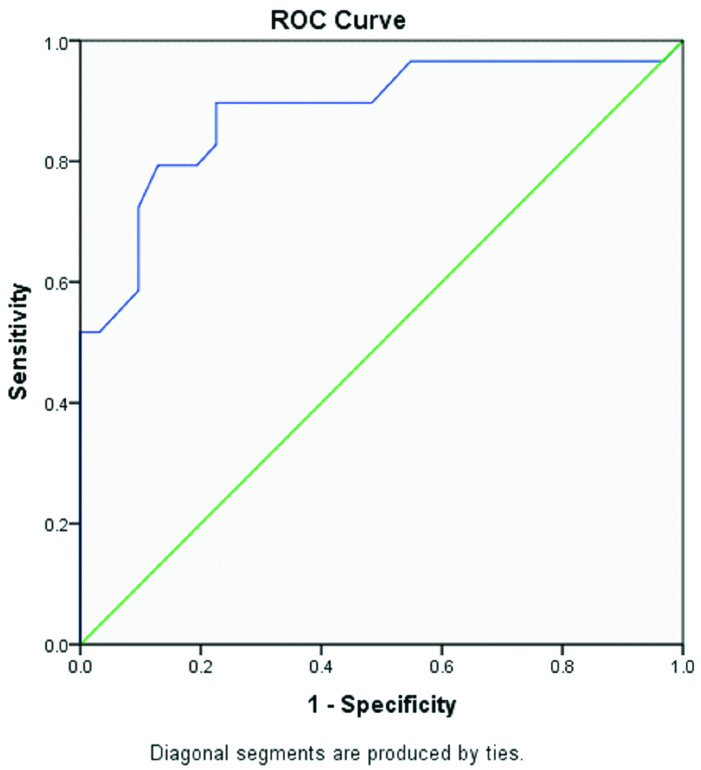
The mean RPR value in mild acute pancreatitis group was 0.038 as compared to 0.068 in the severe acute pancreatitis group. In ROC curve analysis it was found that at a cut of value of 0.045 RPR has a sensitivity of around 90% and specificity of around 73% in predicting the severity of the disease [Table/Fig-4].
ROC curve of RPR as an indicator of disease severity in patients with AP.
A high RPR value was found to be significantly associated with a negative outcome (p-value <0.001). Like RPR, all other severity scores were also found to be significantly elevated in patients with poor outcome including the CRP value; but the association of age was found to be nonsignificant with the disease outcome.
Discussion
There are number of scoring systems developed to predict outcome in acute pancreatitis. Scoring systems like APACHE II, SOFA are cumbersome for calculation. For RANSON and Glasgow score we have to wait for 48 hours. BISAP score is a clinical score, which is based on routine investigations and can be repeated easily and it is a better score for continuous vigilance. But still it requires multiple variables for calculation [20]. CT scoring has similar accuracy as the clinical scores and not recommended solely as a prognostic tool in acute pancreatitis [21]. C-reactive protein [22], haematocrit level [23,24], procalcitonin [25,26] and urinary trypsinogen activating peptide [27] level; all were used as a single test marker of severity of acute pancreatitis with variable results in different study. But, it will be better if we find out a marker from routine investigations, done in every case, so that we can better use our resources.
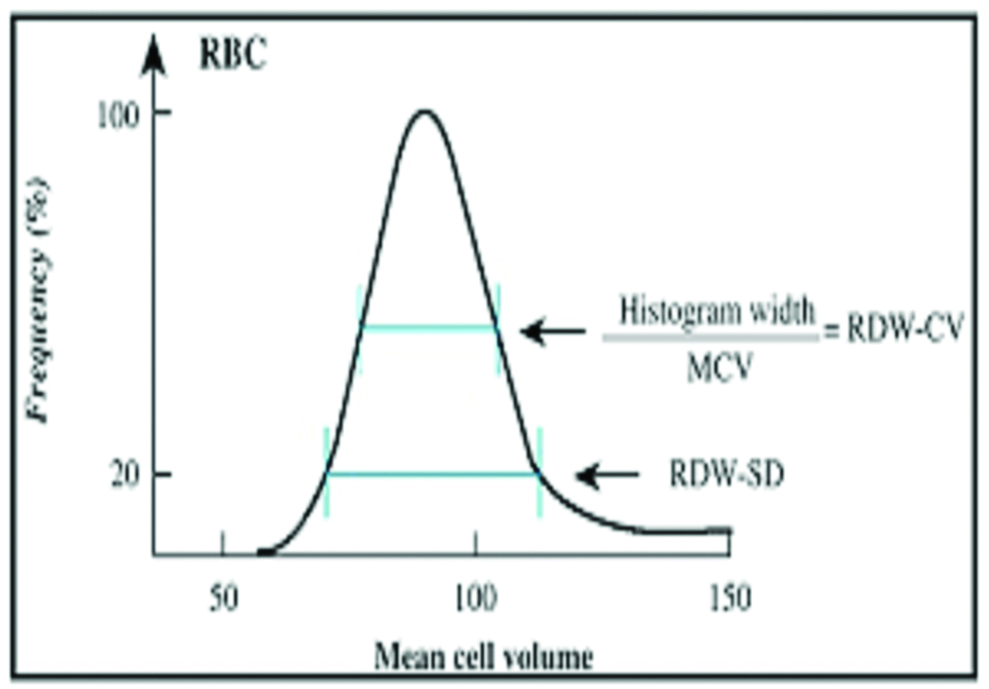
RDW is a blood cell parameter that measures the degree of anisocytosis in red blood cells. There are two ways of measuring RDW, as coefficient of variation (RDW-CV) or as Standard Deviation (RDW-SD) [15]. RDW-SD (expressed in fL) is an actual measurement of the width of the RBC size distribution histogram and is measured by calculating the width of the curve (in fL) at the 20% height level of the RBC size distribution histogram [Table/Fig-5]. The RDW-CV is calculated from the width of the histogram at 1 SD from the mean divided by MCV. The normal RDW-CV is 11.5% to 14.5% and the normal RDW-SD is 39 to 47 fL. We used RDW-CV for our calculation.
Calculation of RDW-CV from the width of the RBC distribution histogram at 1 SD from the mean divided by MCV.
RDW is a reflection of degree of anisocytosis. It gets deranged in haemolytic disorders, in chronic inflammatory disorders, in chronic liver, kidney and lung disorders and in some carcinomas, under the effect of various cytokines [28].
RDW has been studied as a prognostic marker in acute pancreatitis by various authors. In a study of 102 patients Senol K et al., demonstrated that an increased RDW level on admission is an independent predictor of mortality in acute pancreatitis patients [29]. Balta S et al., also obtained similar result in 2013 [30]. Wang D et al., confirmed the finding that high RDW value is associated with more mortality in patients with acute pancreatitis [31]. They found the optimal cut-off value to predict death was 14.35 (sensitivity=88.2%, specificity=91.8%).
Many studies have suggested platelet activation in the pathogenesis of acute pancreatitis [32]. Akbal E et al., studied different platelet parameters in 24 acute pancreatitis patients [33]. They found a negative correlation between progression, thrombocyte counts, haemoglobin and Mean Platelet Volume (MPV). Maeda K et al., found an association of Disseminated Intravascular Coagulation (DIC) markers with outcome of acute pancreatitis [34].
Cetinkaya E et al., studied RDW/TPC ratio in 102 patients and found it as a promising prognostic factor in acute pancreatitis [35]. They found both RDW and RPR as independent and significant variables on admission to predict mortality. According to them, the median RPR was higher in the non-survivor group (0.000087, they have taken TPC as total counts/μL) than in the survivor group (0.000058). They conclude that, RPR with a cut-off value of 0.000067 could predict the mortality in approximately 80% of the patients. In our study, we took the ratio of RDW-CV % to TPC in x1000/μL ratio shortly as RPR; as potential marker of severity in acute pancreatitis. In our study of 60 patients, RPR was consistently found in severe cases and also in the non-survivor group. With a cut-off value of 0.071, RPR as a predictor of mortality in acute pancreatitis was found to have a sensitivity of around 82% and specificity of around 96% and with a cut-off value of 0.045 the RPR is having a sensitivity of around 90% and specificity of around 73% in predicting the severity of the disease.
As complete blood count is a routine investigation done in all indoor admission patients, so if a variable from complete blood count like RPR can predict the disease severity in acute pancreatitis early, then it will be definitely helpful for us to decide in which patients more detailed investigations or extra care is required and we can use our resources accordingly. From this study we confirmed that RPR is a good predictor of disease severity in patients with acute pancreatitis.
Limitation
Number of patients included in the study were less. The present findings needs to be confirmed in larger studies before it can be used in clinical practice.
Conclusion
The results of the study are very promising. So RPR on admission can be used to stratify the severity in acute pancreatitis patients and use of resources can be directed accordingly. Patients with a high RPR value should be transferred to an intensive care unit and frequent monitoring of the condition is required in these patients.
[1]. Somashekar GK, Amrit KK, Hart A, Alice H, Conwell DL, The changing epidemiology of acute pancreatitis hospitalizations: A decade of trends and the impact of chronic pancreatitisPancreas 2017 46(4):482-88.10.1097/MPA.000000000000078328196021 [Google Scholar] [CrossRef] [PubMed]
[2]. Negi N, Mokta J, Sharma B, Sharma R, Jhobta A, Bodh V, Clinical profile and outcome of acute pancreatitis: A hospital based prospective observational study in sub Himalayan StateJAPI 2018 66:22-24. [Google Scholar]
[3]. Sharma S, Salim M, Gothwal SR, A study on acute pancreatitis-incidence, prevalence, morbidity and mortality, in Western RajasthanIJBAMR 2017 6(3):545-48. [Google Scholar]
[4]. Yang AL, Vadhavkar S, Singh G, Omary MB, Epidemiology of alcohol-related liver and pancreatic disease in the United StatesArch Intern Med 2008 168:649-56.10.1001/archinte.168.6.64918362258 [Google Scholar] [CrossRef] [PubMed]
[5]. Carroll JK, Herrick B, Gipson T, Lee SP, Acute pancreatitis: Diagnosis, prognosis, and treatmentAm Fam Physician 2007 75(10):1513-20. [Google Scholar]
[6]. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Classification of acute pancreatitis 2012: revision of the Atlanta classification and definitions by international consensusGut 2013 62:102-11.10.1136/gutjnl-2012-30277923100216 [Google Scholar] [CrossRef] [PubMed]
[7]. Phillip V, Steiner JM, Algül H, Early phase of acute pancreatitis: Assessment and managementWorld J Gastrointestinal Pathophysiology 2014 5(3):158-68.10.4291/wjgp.v5.i3.15825133018 [Google Scholar] [CrossRef] [PubMed]
[8]. Bradley EL III, A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992Arch Surg 1993 128:586-90.10.1001/archsurg.1993.014201701220198489394 [Google Scholar] [CrossRef] [PubMed]
[9]. Kwong WT, Ondrejková A, Vege SS, Predictors and outcomes of moderately severe acute pancreatitis. Evidence to ReclassifyPancreatology 2016 16(6):94094510.1016/j.pan.2016.08.00127618656 [Google Scholar] [CrossRef] [PubMed]
[10]. Seth HS, Mishra P, Khandekar JV, Raut C, Mohapatra CKR, Ammannaya GK, Relationship between high red cell distribution width and systemic inflammatory response syndrome after extracorporeal circulationBraz J Cardiovasc Surg 2017 32(4):288-94.10.21470/1678-9741-2017-002328977201 [Google Scholar] [CrossRef] [PubMed]
[11]. Pierce CN, Larson DF, Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist devicePerfusion 2005 17:83-90.10.1191/0267659105pf793oa15918445 [Google Scholar] [CrossRef] [PubMed]
[12]. Semeraro N, Ammollo CT, Semeraro F, Colucci M, Sepsis-associated disseminated intravascular coagulation and thromboembolic diseaseMediterr J Haematol Infect Dis 2010 2(3):e201002410.4084/mjhid.2010.02421415977 [Google Scholar] [CrossRef] [PubMed]
[13]. Dewitte A, Lepreux S, Villeneuve J, Rigothier C, Combe C, Ouattara A, Blood platelets and sepsis pathophysiology: A new therapeutic prospect in critical ill patientsAnn Intensive Care 2017 7(1):11510.1186/s13613-017-0337-729192366 [Google Scholar] [CrossRef] [PubMed]
[14]. Working Group IAP/APA Acute Pancreatitis GuidelinesIAP/APA evidence-based guidelines for the management of acute pancreatitisPancreatology 2013 13(4 Suppl 2):e1-e15.10.1016/j.pan.2013.07.063 [Google Scholar] [CrossRef]
[15]. Yenilmez ED, Tuli A, Laboratory approach to anemiaCurrent Opinions in Anemia 2018 12:242-43.10.5772/intechopen.70359 [Google Scholar] [CrossRef]
[16]. Basit H, Ruan GJ, Mukherjee S, Ranson CriteriaIn: StatPearls [Internet] 2019 Treasure Island (FL)StatPearls PublishingAvailable from: https://europepmc.org/books/NBK482345;jsessionid=EC59DDABBC82C1ACE253E3945F8697DA [Google Scholar]
[17]. Leese T, Shaw D, Comparison of three Glasgow multifactor prognostic scoring systems in acute pancreatitisBr J Surg 1988 75(5):460-62.10.1002/bjs.18007505193390678 [Google Scholar] [CrossRef] [PubMed]
[18]. Suvarna R, Pallipady R, Bhandary N, Hanumanthappa , The clinical prognostic indicators of acute pancreatitis by APAChe II ScoringJCDR 2011 5(3):459-63. [Google Scholar]
[19]. Tee YS, Fang HY, Kuo IM, Lin YS, Huang SF, Yu MC, Serial evaluation of the SOFA score is reliable for predicting mortality in acute severe pancreatitisMedicine (Baltimore) 2018 97(7):e965410.1097/MD.000000000000965429443733 [Google Scholar] [CrossRef] [PubMed]
[20]. Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Johannes RS, A prospective evaluation of the bedside index for severity in acutepancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitisAm J Gastroenterology 2009 104:966-71.10.1038/ajg.2009.2819293787 [Google Scholar] [CrossRef] [PubMed]
[21]. Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitisAm J Gastroenterology 2012 107(4):612-19.10.1038/ajg.2011.43822186977 [Google Scholar] [CrossRef] [PubMed]
[22]. Cardoso FS, Ricardo LB, Oliveira AM, Canena JM, Horta DV, Papoila AL, C-reactive protein, prognostic accuracy in acute pancreatitis: timing of measurement and cut-off pointsEuro J Gastroenterology Hepatology 2013 25(7):784-89.10.1097/MEG.0b013e32835fd3f023492986 [Google Scholar] [CrossRef] [PubMed]
[23]. Gan SI, Romagnuolo J, Admission haematocrit: a simple, useful and early predictor of severe pancreatitisDig Dis Sci 2004 49(11-12):1946-52.10.1007/s10620-004-9598-815628731 [Google Scholar] [CrossRef] [PubMed]
[24]. Remes-Troche JM, Duarte-Rojo A, Morales G, Robles-Díaz G, Hemoconcentration is a poor predictor of severity in acute pancreatitisWorld J Gastroenterology 2005 11(44):7018-23.10.3748/wjg.v11.i44.701816437609 [Google Scholar] [CrossRef] [PubMed]
[25]. Dias BH, Rozario AP, Olakkengil SA, Procalcitonin strip test as an independent predictor in acute pancreatitisIndian J Surg 2015 77(Suppl 3):1012-17.10.1007/s12262-014-1112-827011501 [Google Scholar] [CrossRef] [PubMed]
[26]. Müller CA, Uhl W, Printzen G, Gloor B, Bischofberger H, Tcholakov O, Role of procalcitonin and granulocyte colony stimulating factor in the early prediction of infected necrosis in severe acute pancreatitisGut 2000 46:233-38.10.1136/gut.46.2.23310644318 [Google Scholar] [CrossRef] [PubMed]
[27]. Khan Z, Vlodov J, Horovitz J, Jose RM, Iswara K, Smotkin J, Urinary trypsinogen activation peptide is more accurate than haematocrit in determining severity in patients with acute pancreatitis: A prospective studyAm J Gastroenterology 2002 97(8):1973-77.10.1111/j.1572-0241.2002.05953.x12190163 [Google Scholar] [CrossRef] [PubMed]
[28]. Salvagno GL, Gomar FS, Picanza A, Lippi G, Red blood cell distribution width: A simple parameter with multiple clinical applicationsCritical Reviews in Clinical Laboratory Sciences 2015 52(2):86-105.10.3109/10408363.2014.99206425535770 [Google Scholar] [CrossRef] [PubMed]
[29]. Şenol K, Saylam B, Kocaay F, Tez M, Red cell distribution width as a predictor of mortality in acute pancreatitisAm J Emerg Med 2013 31:687-89.10.1016/j.ajem.2012.12.01523399348 [Google Scholar] [CrossRef] [PubMed]
[30]. Balta S, Demirkol S, Cakar M, Ardic S, Celik T, Demirbas S, Red cell distribution width: a novel and simple predictor of mortality in acute pancreatitisAm J Emerg Med 2013 31(6):991-92.10.1016/j.ajem.2013.02.03723602743 [Google Scholar] [CrossRef] [PubMed]
[31]. Wang D, Yang J, Zhang J, Zhang S, Wang B, Wang R, Red cell distribution width predicts deaths in patients with acute pancreatitisJ Res Med Sci 2015 20(5):424-28.10.4103/1735-1995.16395126487869 [Google Scholar] [CrossRef] [PubMed]
[32]. Osada J, Wereszczynska-Siemiatkowska U, Dabrowski A, Dabrowska MI, Platelet activation in acute pancreatitisPancreas 2012 41(8):1319-24.10.1097/MPA.0b013e31824bd89f22617709 [Google Scholar] [CrossRef] [PubMed]
[33]. Akbal E, Demirci S, Koçak E, Köklü S, Başar O, Tuna Y, Alterations of platelet function and coagulation parameters during acute pancreatitisBlood Coagulation Fibrinolysis 2013 24(3):243-46.10.1097/MBC.0b013e32835aef5123425662 [Google Scholar] [CrossRef] [PubMed]
[34]. Maeda K, Hirota M, Ichihara A, Ohmuraya M, Hashimoto D, Sugita H, Applicability of disseminated intravascular coagulation parameters in the assessment of the severity of acute pancreatitisPancreas 2006 32(1):87-92.10.1097/01.mpa.0000186248.89081.4416340749 [Google Scholar] [CrossRef] [PubMed]
[35]. Cetinkaya E, Senol K, Saylam B, Tez M, Red cell distribution width to platelet ratio: New and promising prognostic marker in acute pancreatitisWorld J Gastroenterology 2014 20(39):14450-54.10.3748/wjg.v20.i39.1445025339831 [Google Scholar] [CrossRef] [PubMed]