Natural products have been widely used as potent and safe neutraceuticals to treat dyslipidemia and its related diseases, such as metabolic syndrome and Cardiovascular Disease (CVD). PCO is a mixture of eight aliphatic primary alcohols refined from sugarcane wax (Saccharum officinarum L). that contains octacosanol (C28) as the main constituent. It has been reported that consumption of PCO can significantly reduce serum TC and Low-density Lipoproteins (LDL)-Cholesterol (LDL-C) levels as well as elevate serum High-Density Lipoproteins (HDL)-Cholesterol (HDL-C) [1]. PCO exhibits anti-ageing and lipid-lowering effects by enhancing the functionality of HDL-C [2]. HDL-C is a potent lipoprotein, a cardioprotective agent with an anti-oxidant activity that lowers cardiovascular mortality and morbidity associated with atherosclerosis, dyslipidemia and diabetes [3]. Therefore, raising HDL-C and improving its functionality instead of lowering LDL-C could be more beneficial in treating dyslipidemia. In rabbit model, PCO significantly increased LDL receptor-dependent processing, which further increased the LDL catabolic rate and thus lowered LDL levels [4]. Previous studies have established that a higher level of serum HDL-C is associated with a lower incidence of coronary artery disease and attenuated ageing [5-7]. In a recent study on humans, the present research group observed that 8 weeks, 12 weeks, or 24 weeks consumption of PCO were efficient to treat pre-hypertension and dyslipidemia [8-10].
SCWA also known as D-003, is a mixture of high aliphatic primary acids (C24-C36) isolated and purified from sugar cane wax (Saccharum officinarum L.), that have antioxidant, cholesterol-lowering, and anti-platelet properties [10-12]. Pérez Y et al., studied that ant-platelet activity of the natural compounds, D-003 and PCO and reported that D-003 prominently inhibited in-vitro cyclooxygenase-1 (COX-1) activity in rat platelet microsomes [13]. Although SCWA plays an important role in lipid metabolism, however its exact molecular mechanism of lipoprotein functionality is yet to be deciphered. In contrast, the atheroprotective properties of PCO with the improvement of dyslipidemia have been well reported by the present research group from both human clinical study and zebrafish animal study model [8, 9, 14].
Based on previous literature, the current study was designed to compare the effectiveness of SCWA and PCO as a therapeutic agent in dyslipidemia and to further understand the underlying lipid-lowering mechanism. In order to compare the atheroprotective effect of PCO and SCWA in vertebrate animals, the present authors used a zebrafish (Danio rerio) model inducted hypercholesterolemia by HCD to mimic early atherogenesis and its complications, as previously reported by the group [14].
Materials and Methods
Wildtype zebrafish and embryos were maintained according to standard protocols [15]. The maintenance of zebrafish (12-week old) and feeding experiment of 10 weeks, from February to April in 2017, was carried out in zebrafish facility of Lipolab in Yeungnam University. Wild-type zebrafish were obtained from the Zebrafish Center for Disease Modeling (ZCDM), Korea. This experimental study was approved by ‘Committee of Animal Care and Use’ Yeungnam University, Gyeongsan, Korea (YU-2016-002).
Both PCO and SCWA were obtained from Rainbow & Nature Pty, Ltd (Thornleigh, NSW, Australia). The quality of both SCWA and PCO was strictly controlled by monitoring contents and ratio of major ingredients in all the batches.
Zebrafish (Preparation of Samples)
The zebrafish were maintained in a system cage at 28°C during treatment under a 12:12 hours light cycle on normal tetrabit diet (Tetrabit Gmbh D49304, 47.5% crude protein, 6.5% crude fat, 2.0% crude fiber, 10.5% crude ash, containing vitamin A (29,770 IU/kg), vitamin D3 (1,860 IU/kg), vitamin E (200 mg/kg) and vitamin C (137 mg/kg); Melle, Germany). The animals were divided into six groups (n=50) namely: Normal Diet (ND) control, HCD, HCD with 40 mg SCWA/kg of Body Weight (BW), HCD with 80 mg SCWA/kg of BW, HCD with 40 mg PCO/kg of BW and HCD with 80 mg PCO/kg of BW. After 10 weeks’ feeding, each zebrafish was anesthetised by submerging in phenoxyethanol solution (final 0.1%). To avoid haemolysis, blood (2 μL) was directly drawn from heart of the zebrafishes using 22G needle. The blood was combined with 5 μL of Phosphate Buffered Saline (PBS)-Ethylenediaminetetraacetic acid (EDTA, final concentration, 1 mM) and then collected in EDTA-treated tubes.
Plasma Analysis
Plasma TC, HDL-C and TG levels were measured using commercially available assay kits (cholesterol, T-CHO, and TG, Cleantech TS-S; Wako Pure Chemical, Osaka, Japan) [16]. Blood glucose, Glutamate-Oxaloacetate Transaminase (GOT) and Glutamate-Pyruvate Transaminase (GPT) were also measured using commercially available assay kits (AM-201, AM-103K, and AM-102) purchased from Asan Pharmaceutical (Hwasung, Korea).
Cholesteryl Ester Transfer Assay
A reconstituted HDL (rHDL) containing apoA-I and Cholesteryl Ester (CE) was synthesised as per protocol designed in the previous study, using trace amounts of (3H)-cholesteryl oleate (TRK886, 3.5 μCi/mg of apoA-I; GE Healthcare) [17]. The rHDL was immobilised on agarose using CNBr-activated Sepharose 4B resin (Amersham Biosciences) as per the manufacturer’s instructions. The CE-transfer reaction was performed in 300-μL reaction mixtures each containing human HDL3 (20 μL, 2 mg/mL) as a cholesteryl ester transfer protein (CETP) source, rHDL-agarose (20 μL, 0.25 mg/mL) as a CE-donor and human LDL (20 μL, 0.25 mg/mL) as a CE-acceptor. SCWA-rHDL or PCO-rHDL was added to the reaction mixture as an inhibitor. After incubation at 37°C for 4 hours, the reaction was halted by brief centrifugation at 10,000 g for three minutes at 4°C to pellet down the r-HDL-agarose. The supernatant containing CE-acceptor (150 μl) was then subjected to scintillation counting, and the percentage transfer of (3H)-CE from rHDL to LDL was calculated.
Immunoblotting
The composition of apolipoprotein/lipoprotein was analysed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Equal amount (5 μg of total protein per lane) of each sample was loaded and the levels of apolipoprotein expression were measured by immunodetection. Anti-apoA-I antibody (ab7613) and anti-CETP antibody (ab19012) were purchased from Abcam (Cambridge, UK). Anti-GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) antibody (AAS79585C; Antibody Verify, Las Vegas, NV, USA) and secondary goat anti-rabbit immunoglobulin G-horseradish peroxidase (SC2004; Santa Cruz, Dallas, TX, USA) were also used. Protein was quantitated by Bradford assay (Bio-Rad, Hercules, CA, USA) before loading equal amounts of protein (5 μg/lane) from each lysate into 15% SDS-PAGE gels. Relative Band Intensity (BI) was compared by densitometry using Chemi-Doc® XRS+ (Bio-Rad, Hercules, CA, USA) and Image Lab software (Version 5.2, Bio-Rad, Hercules, CA, USA). Blot images were imported into Quantity One software and the contrast was adjusted to make the bands fully noticeable on the blot image.
Histologic Analysis
The zebrafish were sacrificed and their livers were fixed in 4% paraformaldehyde for 24 hours. These fixed tissues were then embedded in Tissue-Tek OCT compound (Thermo, Walldorf, Germany). The frozen tissue blocks were sectioned using cryomicrotome analyzer (Leica CM1510S, Nussloch, Germany). Seven consecutive sectioned slides from each zebrafish were then stained with Oil Red O to detect the lipid accumulation in the liver tissue.
To compare inflammatory responses in tissues, levels of Reactive Oxygen Species (ROS) were imaged using Dihydroethidium (DHE, cat. no. 37291, Sigma, St. Louis, MO) following microtome sectioning as previously described [18]. The image was obtained by fluorescence (Ex=588 nm and Em=615 nm) using a Nikon Eclipse TE2000 microscope (Tokyo, Japan). Section fluorescence was quantified via computer-assisted morphometry using Image Proplus software (v. 4.5.1.22, Media Cybernetics, Bethesda, MD).
Aliquots of hepatic tissue from each zebrafish were homogenised for 3 minutes (150 rpm) in an ice bath using a tissue homogenizer (Euro-ST; Eurostar, IKAWERKE, Staufen, Germany). After brief centrifugation at 10,000 x g, the protein content was estimated using Bradford reagent and equally diluted supernatants (1 mg/mL of total protein) were used for determination of oxidative species using the Thiobarbituric Acid Reactive Substances (TBARS) method. The supernatant (0.2 mL) was subjected to TBARS assay to compare levels of oxidised species in the hepatic tissue using a Malondialdehyde (MDA) standard [19].
Ferric Reducing Ability Assay
The FRA of hepatic tissue homogenate was determined using the method described by Benzie IF and Strain JJ [20]. The antioxidant activities of individual homogenate fractions (20 μg each in, PBS) were estimated by measuring increase in absorbance induced by generated ferrous ions.
Statistical Analysis
All data were expressed as the mean±SD (Standard Deviation) from three independent experiments with duplicate samples. Data were evaluated via one-way analysis of variance (ANOVA) using Statistical Software for Social Sciences (SPSS) version 23.0 (SPSS, Inc., Chicago, IL, USA), and the differences between the means were assessed using Duncan’s multiple-range test. Statistical significance was defined at p<0.05.
Results
Enhanced Survivability
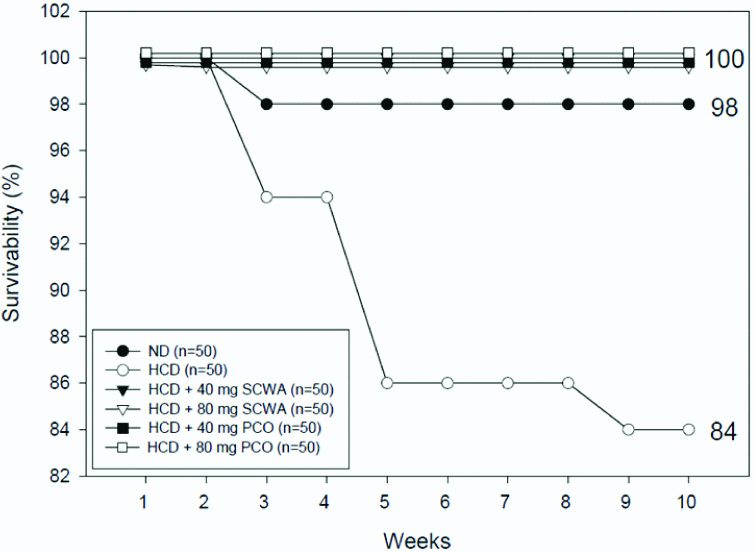
After 10 weeks supplementation, ND and HCD control groups showed 98% and 84% survival, respectively. The survivability of HCD groups dropped to 86% at 5 weeks consumption, suggesting that hyperlipidemia caused sudden death. In comparison, the other four groups under HCD, 40 mg SCWA/kg of BW, 80 mg SCWA/kg of BW, 40 mg PCO/kg of BW, and 80 mg PCO/kg of BW, showed 100% survival during 10 weeks [Table/Fig-1].
Survivability of zebrafish during 10 weeks supplementation of either Sugarcane Wax Acid (SCWA) or Policosanol (PCO); HCD: High Cholesterol Diet; ND: Normal Diet
Blood Lipid Lowering Effect
Body weight and serum biochemical profile of zebrafish supplemented with either SCWA (40 mg SCWA/kg of BW, and 80 mg SCWA/kg of BW) or PCO (40 mg PCO/kg of BW and 80 mg PCO/kg of BW) were assessed [Table/Fig-2]. After 10 weeks of consumption, HCD control groups showed a 9.5% increase in BW as compared to ND group. Under HCD, PCO group and SCWA group also showed increase of BW except SCWA 80 mg group, which showed similar BW with ND group, indicating that the high-dose of SCWA might have anti-obesity effect. HCD group showed 2.5-fold and 2.3-fold elevated TC and TG levels, respectively, than ND group during 10 weeks supplementation. SCWA (80 mg) group showed the least increase in TC and TG levels. Compared to HCD control, high dose of SCWA or PCO group also showed significantly reduced serum TC upto 65% or 40%, respectively. TG levels in different groups were significantly decreased compared with HCD control (40 mg SCWA, 80 mg SCWA, 40 mg PCO, 80 mg PCO) by 35%, 44%, 31% and 41%, respectively. The maximum reduction of glucose, up to 41%, was observed in 80 mg SCWA/kg of BW group compared with HCD control. The HDL-C concentration in high dose SCWA and PCO group were significantly elevated compared to HCD control. The Percentage (%) HDL-C was also higher in the high-dose groups of SCWA and PCO up to 65% and 47%, respectively. The % CE-transfer activity was decreased by 47% and 50% in high dose of SCWA and PCO group, respectively, compared to the HCD control group. The liver enzyme GOT was significantly reduced by 41% by SCWA and by 23% by PCO compared with HCD control. The GPT levels in SCWA and PCO groups also showed similar reduction trends against the HCD control.
Serum lipid profile of zebrafish after 10 weeks supplementation of either Sugarcane Wax Acid (SCWA) or policosanol (PCO).
| Diet group | ND1 Control | HCD2 Control | HCD+40 mg/kg SCWA | HCD+80 mg/kg SCWA | HCD+40 mg/kg PCO | HCD+80 mg/kg PCO |
|---|
| Week 0 (n) | 50 | 50 | 50 | 50 | 50 | 50 |
|---|
| Week 10 (n) | 49 | 42 | 50 | 50 | 50 | 50 |
|---|
| BW (mg), week 0 | 356±69 | 398±90 | 397±78 | 385±72 | 367±70 | 354±94 |
| BW (mg), week 10 | 627±60 | 687±71 | 703±71 | 628±65* | 676±60 | 661±62 |
| TC (mg/dL) | 242±10 | 620±32 | 408±35** | 218±15*** | 461±41** | 378±35** |
| TG (mg/dL) | 98±13 | 223±25 | 145±19* | 124±13* | 154±10* | 132±14* |
| Glucose (mg/dL) | 84±16 | 90±23 | 57±12* | 53±15* | 65±20* | 61±18* |
| GOT (U/L) | 109±12 | 232±24 | 142±12** | 137±12** | 193±17* | 178±18* |
| GPT (U/L) | 55±6 | 63±6 | 58±7* | 55±5* | 56±6* | 55±7* |
| HDL-C (mg/dL) | 31±5 | 45±3 | 51±6 | 58±3* | 53±3* | 64±3** |
| % HDL-C in TC | 13±2 | 7±2 | 10±2 | 26±4* | 11±2 | 17±3* |
| % CE-transfer | 11±2 | 32±4 | 25±4* | 18±2* | 23±3* | 16±3* |
1 ND: normal diet; Tetrabit®: Tetrabit (47.5% crude protein, 6.5% crude fat, 2.0% crude fiber, 10.5% crude ash, containing vitamin A [29,770 IU/kg]; vitamin D3 [1860 IU/kg]; vitamin E [200 mg/kg]; and vitamin C [137 mg/kg]); 2 HCD: high cholesterol diet; Tetrabit + 4% cholesterol; BW: Body Weight; CE: Cholesteryl Ester; GOT: Glutamate Oxaloacetate Transaminase; GPT: Glutamate Pyruvate Transaminase; HDL-C: High-Density Lipoproteins-Cholesterol; PCO, Policosanol; SCWA: Sugar Cane Wax Acid; TC: Total Cholesterol; TG: Triglycerides Differences with a p-value <0.05 were considered significant; *: p<0.05 vs; HCD control at week 10; **: p<0.01 vs. HCD control week 10; ***: p<0.001 vs. week 10
Enhancement of apoA-I Expression
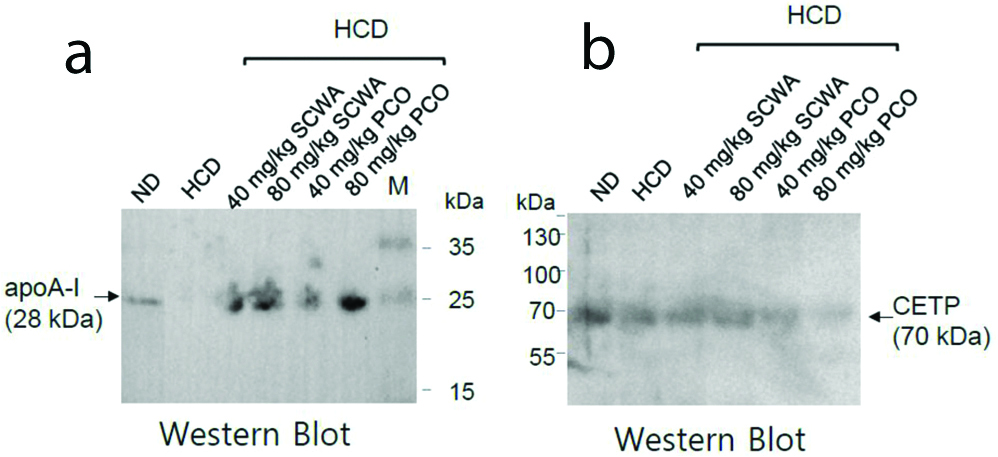
Immunoblot analysis was performed on 15% SDS-PAGE to measure the expression levels of apoA-I and CETP [Table/Fig-3]. Expressional level of the apoA-I was higher in the SCWA or PCO (80 mg/kg of BW) group than in the HCD alone group [Table/Fig-3a]. ApoA-1 expression level was similar in PCO 80 mg/kg of BW group compared to SCWA 80 mg/kg of BW group. The enhanced expression of apoA-I in zebrafish fed SCWA or PCO clearly indicates that HDL functionality was improved [Table/Fig-3b]. The expression of CETP was lower in the 80 mg PCO group compared with the SCWA 80 mg or HCD alone group. These results suggest that the higher dose of PCO inhibits the expression of CETP, and therefore can be used as CETP inhibitors in-vivo.
Immuno-detection of apoA-I (a) and CETP (b) in zebrafish plasma.
Amelioration of Fatty Liver Change
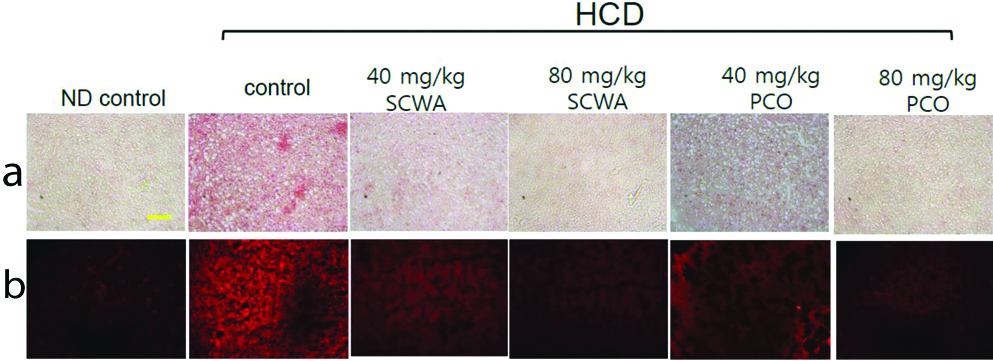
The [Table/Fig-4a,b] shows the histologic analysis of hepatic tissue stained with Oil Red O stain (a) and DHE staining (b). Oil Red O staining revealed a remarkable increase in red intensity in the HCD group compared with normal group, indicating that high cholesterol consumption induced fat accumulation in the liver. The tissue staining also revealed that the 80 mg PCO and 80 mg SCWA groups had low fat deposition in the liver (less intense red colour after Oil Red O staining). DHE staining showed that ROS production was highest in the HCD group compared with SCWA and PCO treated groups [Table/Fig-4b]. HCD, 80 mg PCO and 80 mg SCWA groups showed remarkably lowered hepatic lipid deposition and ROS production in the liver.
Histologic analysis of hepatic tissue after supplementation of either Sugarcane Wax Acid (SCWA) or Policosanol (PCO). Representative photos for comparison of fatty liver changes in zebrafish as visualised by Oil Red O staining (a) and Reactive Oxygen Species (ROS) production as visualised by dihydroethidium (DHE) staining (b). Yellow scale bar indicates 100 μm.
Enhanced Anti-Oxidant Ability
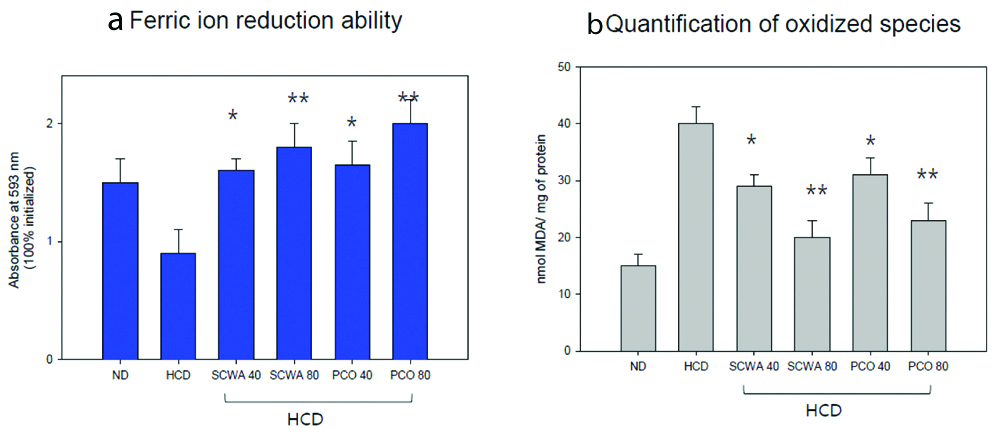
The Ferric Ion Reduction Ability (FRA) of hepatic tissue was elevated in both SCWA and PCO groups in a dose-dependent manner. Groups supplemented with high-dose of PCO and SCWA group showed the highest FRA activity as compared to the HCD control [Table/Fig-5a,b]. Malondialdehyde (MDA) content was also reduced in a dose-dependent manner, 80 mg SCWA and 80 mg PCO group showed the least amount of MDA compared to their respective 40 mg SCWA and 40 mg PCO group and HCD control [Table/Fig-5b].
Anti-oxidant ability of homogenate of hepatic tissue from each group. Anti-oxidant ability of homogenate of hepatic tissue from each group. (a) Ferric ion reduction ability of hepatic tissue; (b) Quantification of oxidised species (malondialdehyde) in hepatic tissue.
*p<0.05 versus HCD; **p<0.01 versus HCD; ND: Normal diet; HCD: High-cholesterol diet
Discussion
In the current study, the present authors evaluated the efficacy of two natural products SCWA and PCO in hyperlipidemic zebrafish over 10 week’s supplementation. Zebrafish have been widely used to evaluate efficacy of putative hyperlipidemic agent because zebrafish has high CETP activity which is very similar to mammalian system. Since normal rat and mouse do not have significant serum CETP activity they remain resistant to induction of hyperlipidemia by a HCD supplementation. High doses group of PCO and SCWA showed lowered body weights compared to the HCD control group. Under HCD, consumption of either SCWA or PCO significantly reduced the serum TC, elevated % HDL-C in TC level and ameliorated the fatty liver changes. These improvements of lipid profiles are due to enhancement of cholesterol efflux by PCO, stimulates expression of ATP-binding cassette transporter ABCA1 [9]. Cholesterol efflux is a key feature of HDL that exerts removal of cholesterol from atherosclerotic plaques in the reverse cholesterol transport pathway. The elevated expression of apoA-I also contributes to the efflux activity, since it is majorly dependent on the configuration of apoA-I [21].
In addition, the 80 mg PCO group showed the highest HDL-C level and the least CE-transfer activity. The CE-transfer activity was reduced by 50% and 44% in PCO and SCWA group, respectively. The elevated expression of apoA-I and the reduced expression of CETP in SCWA and PCO group indicates that lipoprotein functionality could be improved in zebrafish by 10 weeks supplementation. These results make a good agreement with the previous reports, where PCO supplementation resulted in elevated apoA-I expression [8]. Although the exact mechanism is still unclear, it is suggested that the apoA-I and HDL can stimulate insulin secretion from pancreatic β-cells, exert anti-diabetic activity, whereas glycated or oxidised apoA-I/HDL cannot [9,22-24].
The histologic analysis of hepatic tissue stained by Oil Red O and DHE revealed that PCO and SCWA ameliorated hepatic lipid deposition and ROS production in the liver. PCO elevated the antioxidant capacity after 10 weeks more than the normal and HCD groups. Oxidised species were the lowest level in the 80 mg PCO and the 80 mg SCWA groups.
Policosanol is an effective natural nutritional supplement used to lower total cholesterol, triglyceride and serum LDL cholesterol levels in humans and animal models [25,26]. A recent report revealed that eight weeks of policosanol consumption in Spontaneously Hypertensive Rats (SHR) remarkably reduced blood pressure, serum aldosterone, and serum TG levels increased HDL-C and ameliorated hepatic inflammation [27]. In the hyperlipidemic zebrafish model, PCO exhibits anti-ageing and tissue regenerative effects by enhancing the functionality of HDL [14]. In the current results, the amelioration of hepatic fatty liver change in SCWA and PCO groups were associated with the enhancement of antioxidant ability of hepatic tissue homogenate. These findings are in agreement with the previous studies [14, 27].
The literature on the role of SCWA as lipid-lowering, anti-platelet and anti-inflammatory agent are scarce [28]. Both natural compounds, PCO and D-003 (SCWA), inhibit the cholesterol synthesis pathway by regulating HMG-CoA reductase, which is a target for statin drugs [29,30]. The mechanism by which PCO lowers lipid levels are widely studied and include enhancing the functionality of HDL, lowering LDL levels, inhibiting cholesteryl ester transfer protein and inhibiting glycation and lipoprotein peroxidation [2,14,31,32]. However, the mechanisms of SCWA have not been fully studied in animal models. Therefore, the current results provide a new aspect of SCWA in relation to lipid-lowering mechanism and protection of fatty liver change. Castaño G et al., compared the effects of PCO and D-003 in a randomised double-blinded study in patients with Type II hypercholesterolemia [33]. Compared to PCO, D-003 therapy for eight weeks effectively lowered LDL-C and TC and increased HDL-C in the patients. This study makes a good agreement with our current results demonstrated that D-003 could be used for treating hypercholesterolemia. In addition, study on male Wistar rats treated with D-003 (5–250 mg/kg) for four weeks also reported that the D-003 was more effective than PCO in alleviating the measured lipid peroxidation markers, such as plasma MDA and total peroxides and MDA concentrations in liver homogenates [31]. The results of the current study corroborated the previous findings, highlighting the effectiveness of SCWA over PCO in lowering lipid levels.
Limitation
While the study is one of the pioneers work to report the association of hypolipidemic effect of SCWA and PCO with higher expression of apoA-I, inhibition of CETP activity and enhancement of hepatic antioxidant activity, it does not compare the in-vivo regulatory effect of apoA-I and CETP between PCO and SCWA under normal diet. Further studies are required to compare HDL particle properties and functionality after consumption of SCWA or PCO in a mammalian model.
Conclusion
The study highlights the in-vivo effects of PCO and SCWA supplementation in the management of dyslipidemia. Both SCWA and PCO supplementation showed a similar physiological effect in lowering cholesterol, elevating % HDL-C and apoA-I expression, inhibiting CETP activity and amelioration of fatty liver change in a dose-dependent manner in the hyperlipidemic zebrafish.
Authors contribution: C.H.K, J.R.K and K.H.C performed experiments; C.H.K, M.A.B., and J.R.K. analysed data and; K.H.C. wrote the manuscript and supervised the whole project.