An Interesting and Rare Case of Urachal Adenocarcinoma Enteric Type with Review of Literature
P Manjusha Mullappali1, Sandhya Sundaram2, Lawrence D Cruze3, S Rajendiran4, T Chandru5
1 Postgraduate, Department of Pathology, Sri Ramachandra Institute of Higher Education and Research (Deemed to be University), Chennai, Tamil Nadu, India.
2 Head, Department of Pathology, Sri Ramachandra Institute of Higher Education and Research (Deemed to be University), Chennai, Tamil Nadu, India.
3 Associate Professor, Department of Pathology, Sri Ramachandra Institute of Higher Education and Research (Deemed to be University), Chennai, Tamil Nadu, India.
4 Professor, Department of Pathology, Sri Ramachandra Institute of Higher Education and Research (Deemed to be University), Chennai, Tamil Nadu, India.
5 Professor, Department of Urology, Sri Ramachandra Institute of Higher Education and Research (Deemed to be University), Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sandhya Sundaram, Head, Department of Pathology, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai-600116, Tamil Nadu, India.
E-mail: sandsrid@gmail.com
The urachus is a tubular structure that connects the bladder and allantois in the embryonic development, which involutes after third trimester. Urachal tumours are extremely rare and accounts of about 0.3-0.7% of all the bladder malignancies. We report here a case of 65-year-old male who was a known case of type II Diabetes, presented with complaints of haematuria for one and a half month. On abdominal ultrasonography and computed tomography, showed a pedunculated mass measuring 3×2 cm in the bladder wall protruding into the bladder. Cystoscopy was done, a papillary growth was noted in the dome of the bladder. Cystoscopically guided biopsy was done which showed enteric type adenocarcinoma. The surgical specimen showed urachus adenocarcinoma of enteric type Stage IIIA in the Sheldon System. This case has six months of follow-up without any recurrence or metastasis.
Allantois, Haematuria, Papillary growth, Urachus
Case Report
A 65-year-old male who was a known case of diabetes since two years which was poorly controlled came up with complaint of intermittent haematuria for one and half months. Clinical examination revealed umbilicus everted with a nodule, it was non tender and no discharge was noted. No abdominal mass was palpated.
On haematological investigation, his hemoglobin was 12.4 gm/dL and other clinicopathological blood parameters were within normal limit, urine microscopy revealed pH-6.5, Red blood cells-plenty, pus cells 4 to 6/HPF. On biochemical investigation his glycated haemoglobin was 14%. The liver function, renal function test and electrolytes were within normal limit.
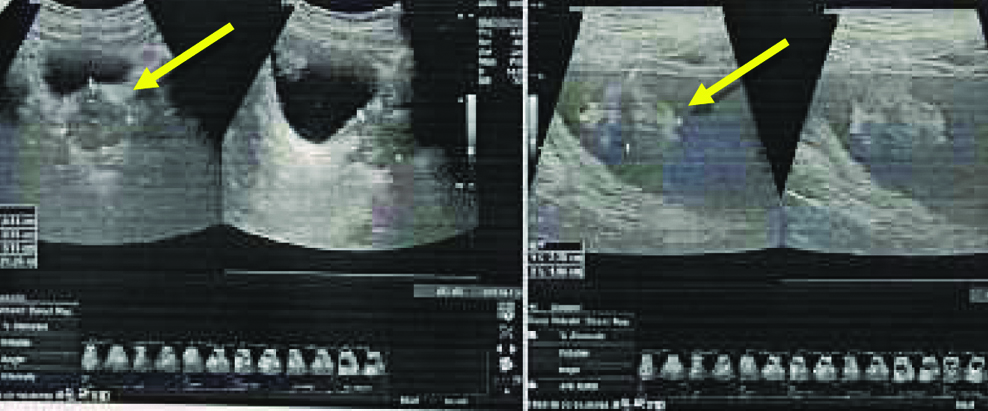
A clinical diagnosis of urothelial carcinoma was suspected in the case and following radiological investigation were done. Ultrasonography showed a pedunculated mass of 2.78×1.96 cm protruding into the bladder [Table/Fig-1]. Computed Tomography confirmed the presence of a heterogeneously enhancing lesion of 2.78×1.96 cm in the dome of bladder, remaining bladder was appearing unremarkable. The clincoradiological probable diagnosis was Urinary bladder cancer arising at dome or a urachal carcinoma and histological correlation was requested.
Ultrasound showing the lesion marked by arrow.
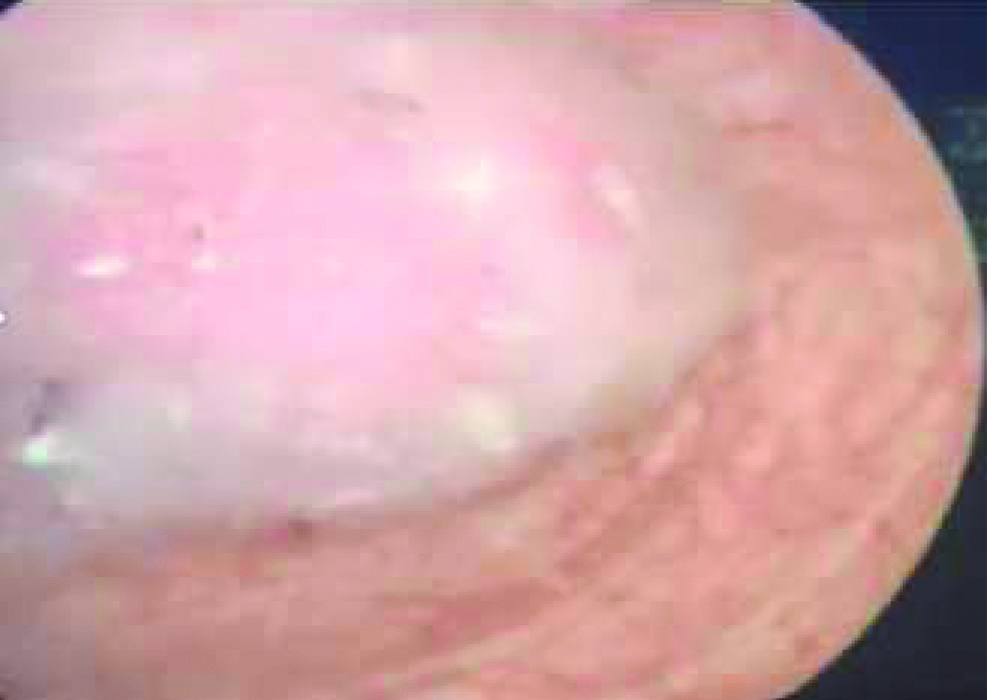
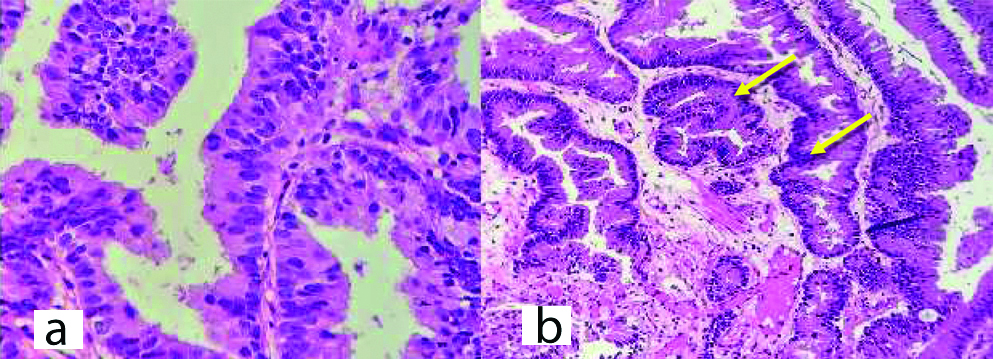
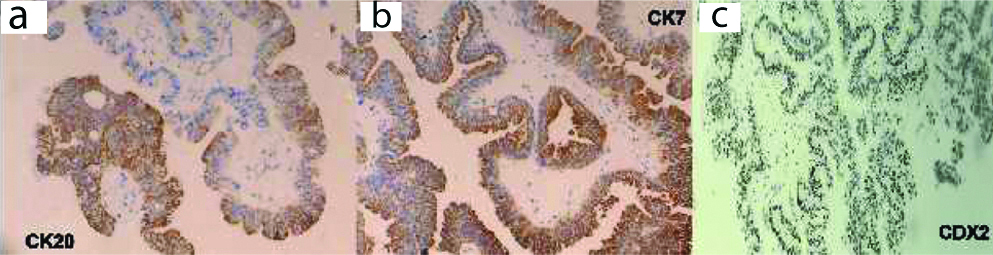
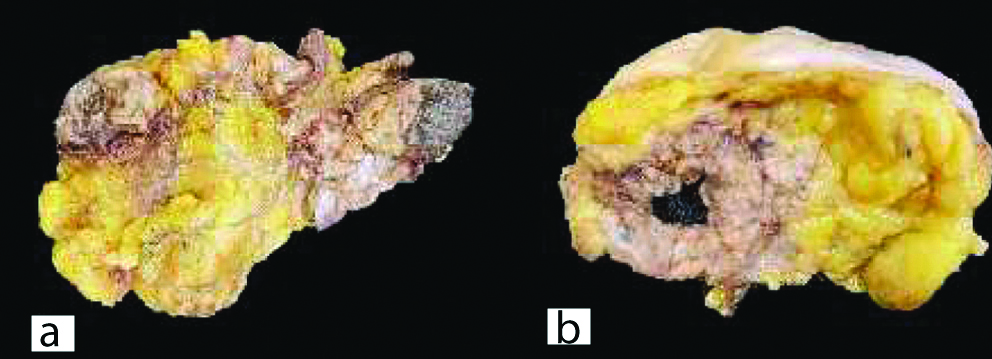
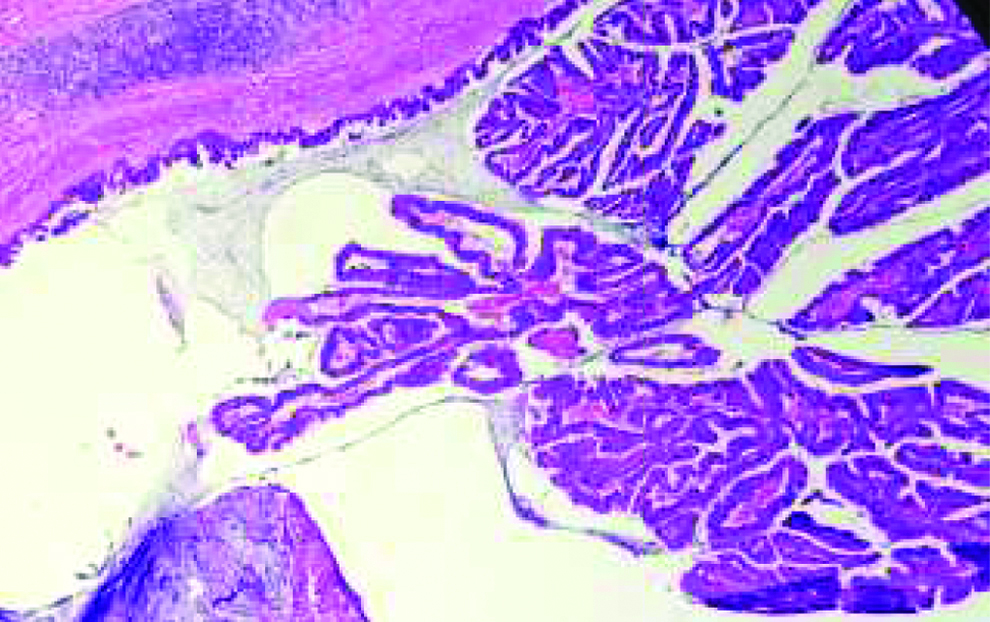
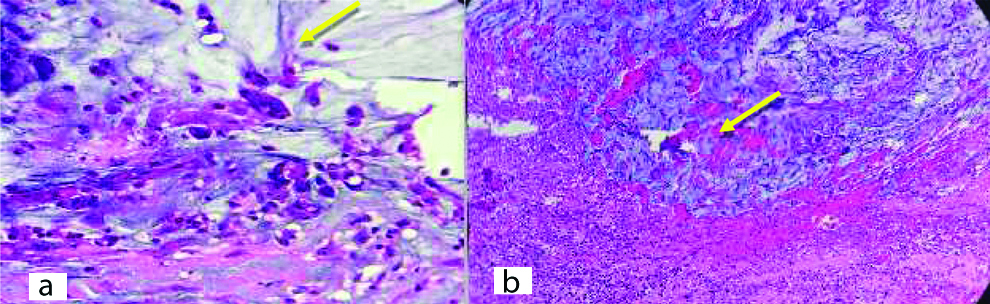
Cystoscopically lesion was papillary growth measuring 3×2 cm located in the dome of the bladder [Table/Fig-2] and transurethral resected biopsy performed and submitted for histopathological examination, grossly received multiple gray white soft tissue fragments and microscopically showed presence of glandular structure showing pseudostratification with mucin pools adjacent to these atypical cells, on higher power of pseudostratification, with cells having increase nuclear cytoplasmic ratio, nuclear atypia [Table/Fig-3] and on immunohistochemical staining, the tumor cells were positive for CK7, CK20 and CDX2 [Table/Fig-4]. A final report of enteric type adenocarcinoma of bladder-urachal origin was suggested following which patient underwent partial cystectomy with umbilectomy. The gross examination of the specimen showed a patent urachus with a lesion arising in the remnant and located on the dome of bladder [Table/Fig-5]. The lesion appeared to be fungating mass measuring around 3×2cm. Histopathological examination [Table/Fig-6], revealed atypical cells lined with glandular structures admixed with mucin seen as acellular pale basophilic secretions in between tumour cells and lower power showed a foci of pseudostratified columnar epithelium with nuclear stratification, nuclear crowding, hyperchromatasia, occasional prominent nucleoli, occasional mitosis and adjacent areas showed the few atypical glands invading into the muscularis propria [Table/Fig-7]. Entire lesion was subjected to examination and no areas of cystitis cystica or glandularis was seen. A diagnosis of adenocarcinoma urachus in background of villous adenoma with high grade dysplasia in urachal remnant was rendered.
Cystoscopy showing a polypoidal lesion in the dome of bladder.
Transurethral resection biopsy-H&E staining (a) Staining showing glandular structure arranged close to each other with stratification. (100X), (b) H&E staining showing pseudostratification, nuclear atypia showed by arrow (400X).
Immunohistochemical stains-Tumor cell positive for CK20 (a), CK7 (b), CDX2 (c). CK7 and CK20 membranous staining and CDX2 nuclear staining.
(a) The entire specimen showing the fungating lesion. (b) Cut surface showed showed a lesion in the dome of the bladder showing 3×2 cm fungating.
H&E stain 100X-partial cystectomy with umbilectomy specimen showing pseudostratification of glandular epithelium in villous architecture, suggestive of villous adenoma.
H&E stain-section from partial cystectomy with umbilectomy-atypical cells and mucin invading the muscularis layer (a) 200X yellow mark shows the atypical cells, (b) 200X shows invasion in the muscularis layer.
Discussion
Urachal tumours are rare tumours arising from the urachal ligament connecting the dome of the bladder inferiorly and superiorly connects via the ligamentum commune to the umbilicus. This tubular structure becomes obliterated by advancing age. But its patency with urinary bladder in small proportion of adults and tumors arising from urachus accounts about 0.3% [1].
These tumours are most commonly seen in patients of 40-70 years of age, two-third of whom are men with complaints with intermittent haematuria [2], our patient was a 65-year-old male with poorly controlled diabetic status, who presented with haematuria which is the usual presentation. To diagnose urachal adenocarcinoma, World Health Organisation (WHO) 2016 textbook has laid down criteria by Gopalan A et al., that the location should be dome of the bladder, epicenter in the bladder wall, absence of cystitis cystica/cystitis glanduralis beyond dome and absence of the primary elsewhere [3]. The present case illustrated here fulfills above criteria’s confirmed by radiological and histologically, these tumours usually extends towards umbilicus.
Normally, urothelium is the lining of urachus but predominant of cases show adenocarcinoma, Two theories proposed for the diagnosis is tumors arising enteric rest during embryogenesis or as a result of metaplasia [2].
According to WHO 2016, the urachal epithelial tumours having adenoma which can be mucinous or villous, adenocarcinoma which can be enteric, mucinous and signet ring cells [4]. The present case showed a well defined villous adenoma with high grade dysplasia resembling colonic epithelium that is enteric variant and a focus showing extracellular acellular pale basophilic secretion. Immunohistochemistry was done which showed CK7, CK20 and CDX2 positive with GATA3 negativity. A small foci of urothelial histology and also presence of cystitis cystica, cystitis glandularis raises a suspicious for non urachal adenocarcinoma. Radiologically, investigation like CT or MRI also aids in diagnosis. Cystoscopy and biopsy of lesion can confirm the adenocarcinoma rather than percutaneous biopsy as the later has a risk of rupture of cystic lesion. Immunohistochemical staining can prove useful in differentiating primary adenocarcinoma from secondary adenocarcinoma from the colon. In bladder adenocarcinoma primary, CK7 and CK20 is positive in contrast to colonic adenocarcinoma which express only CK20 [5]. There is no TNM staging given by College of American Pathologist, we followed the Sheldon Staging system, Stage I -Carcinoma confined urachal carcinoma, II-carcinoma invasion confined to urachus, III-local carcinoma extension which is subclassified as IIIA to bladder, IIIB extension to abdominal wall, IIIC extension to peritoneum and IIID to adjacent viscera, IV is metastasis [6]. Unfortunately, most of the patient with urachal carcinoma present by stage III/IV as in this case was corresponding to the Stage IIIA.
The advance urachal carcinoma accompaines with high risk of distant metastasis. This case was followed up and PET scan was done which showed no recurrence or metastasis.
Surgical resection (partial cystectomy with umbilectomy and bilateral pelvic lymph-node dissection) is main stay and gold standard management which was management for the present case. Like its colonic counterpart, microsatellite instability and KRAS mutations is also reported for urachal adenocarcinoma [7]. At the end of six month of follow-up the patient is doing well.
Conclusion
In summary, we document this rare case of urachal adenocarcinoma, of enteric type arising in the back ground of villous adenoma of the urachus. We also stress the importance of thorough sampling to rule out invasive lesions of the urachus. Further radiology may not clearly delineate these lesions. A new set of criteria had been adapted from Gopalan A et al., and published in the 2016 WHO blue book. Relatively to the outcome there are some studies reporting better survival rates of urachal adenocarcinoma when compared to urothelial carcinoma.
[1]. Collins DC, Velázquez-Kennedy K, Deady S, Brady AP, Sweeney P, Power DG, National incidence, management and survival of urachal carcinomaRare Tumors 2016 8(3):97-101.10.4081/rt.2016.625727746878 [Google Scholar] [CrossRef] [PubMed]
[2]. Siefker-Radtke AO, Urachal and non-urachal adenocarcinomas of the bladderIn rare genitourinary tumors 2016 Springer, Cham:139-151.10.1007/978-3-319-30046-7_9 [Google Scholar] [CrossRef]
[3]. Gopalan A, Sharp DS, Fine SW, Tickoo SK, Herr HW, Reuter VE, Urachal carcinoma: A clinicopathologic analysis of 24 cases with outcome correlationAm J Surg Pathol 2009 33(5):659-68.10.1097/PAS.0b013e31819aa4ae19252435 [Google Scholar] [CrossRef] [PubMed]
[4]. Paner GP, Lopez-Beltran A, Sirohi D, Amin MB, Updates in the pathologic diagnosis and classification of epithelial neoplasms of urachal originAdv Anat Pathol 2016 23(2):71-83.10.1097/PAP.000000000000011026849813 [Google Scholar] [CrossRef] [PubMed]
[5]. Singh I, Prasad R, Primary urachal mucinous adenocarcinoma of the urinary bladderJ Clin Diagn Res 2013 7(5):911-13.10.7860/JCDR/2013/5597.297323814741 [Google Scholar] [CrossRef] [PubMed]
[6]. Sheldon CA, Clayman RV, Gonzalez R, Williams RD, Fraley EE, Malignant urachal lesionsJ Urol 1984 131(1):01-08.10.1016/S0022-5347(17)50167-6 [Google Scholar] [CrossRef]
[7]. Sirintrapun SJ, Ward M, Woo J, Cimic A, High-stage urachal adenocarcinoma can be associated with microsatellite instability and KRAS mutationsHum Pathol 2014 45(2):327-30.10.1016/j.humpath.2013.09.00824355196 [Google Scholar] [CrossRef] [PubMed]