History and Discovery
Apelin Receptor Structure and Properties
In 1993, O’Dowd BF et al., discovered a type of receptor protein and named it Apelin Receptor (APJ/AR) [1]. They characterised a 700 base pair sequence of the Polymerase Chain Reaction (PCR) fragment, which encoded a protein of 380 amino acids protein. The APJ gene (Gene symbol APLNR) is located on chromosome 11q12 and does not contain intron. APJ receptor resembles Angiotensin Type 1 receptor (AT1). APJ and AT1 have structural homology of 115 amino acids (30%) and 86 amino acids in the transmembrane regions (54%) [2].
APJ receptor contains seven hydrophobic transmembrane regions characteristic of G-Protein Coupled Receptor (GPCR) family [2]. GPCR is activated by neuropeptides, polypeptide hormones and non-peptides such as biogenic amines, nucleotides, lipids and ions [3]. Some novel GPCRs do not have their endogenous natural ligands, and are termed as “orphan receptors”. The cAMP-dependent protein kinase phosphorylates the APJ receptor at its consensus sites for palmitoylation and glycosylation.
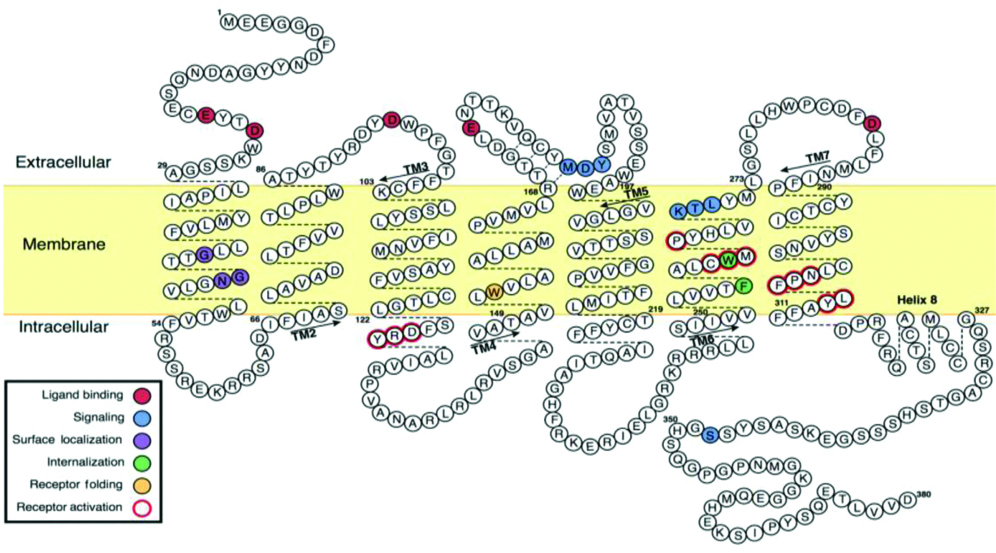
The glycosylation of N-terminal GPCR, has been implicated in the expression, correct folding of nascent protein, stability and ligand binding of receptor [4]. Studies on the APJ receptor structure showed that amino acids in the N-terminal (e.g., Asp23 and Glu20) and C-terminal portions of the APJ receptor are required for internalisation [5,6] [Table/Fig-1].
Human apelin receptor (APJ/AR) structure, with seven delineated transmembrane (TM) helices [6].
Regulation of APJ (APLNR) Gene
The regulation of APJ gene expression has not been extensively studied till date. At the transcriptional level, the highest, rat APJ gene promoter activity is found between -966 and -165 bp. Electrophoretic mobility shift, super-shift and competitive immunoassays have indicated that promoter is under complex regulation by specificity protein 1 (Sp1), oestrogen receptor, glucocorticoid receptor and CCAAT enhancer binding protein γ (C/EBP-γ or CEBPG) transcription factors, with Sp1 as a major regulator of rat APJ gene promoter activity [7]. Various studies on Single Nucleotide Polymorphisms (SNPs) of APLNR gene and reported an association between SNP (rs9943582 (G/A) in 5’ flanking region of Sp1 binding site of APLNR gene with susceptibility to brain infarction [8,9]. Two APLNR gene polymorphisms, in Han Chinese population, rs7119375 (G/A) and rs10501367 (G/A), in Italian patients, rsG212A, suggests association of SNPs with hypertension [10]. In animal model, up-regulation of APJ gene, in response to acute and repeated stress is observed and these changes are likely to be dependent on glucocorticoids [11]. The endogenous ligand, apelin also regulates APJ expression within the Gastrointestinal (GI) tract and up-regulation of APJ expression was observed in animal adipose tissue by insulin [12].
Apelin Structure and Properties
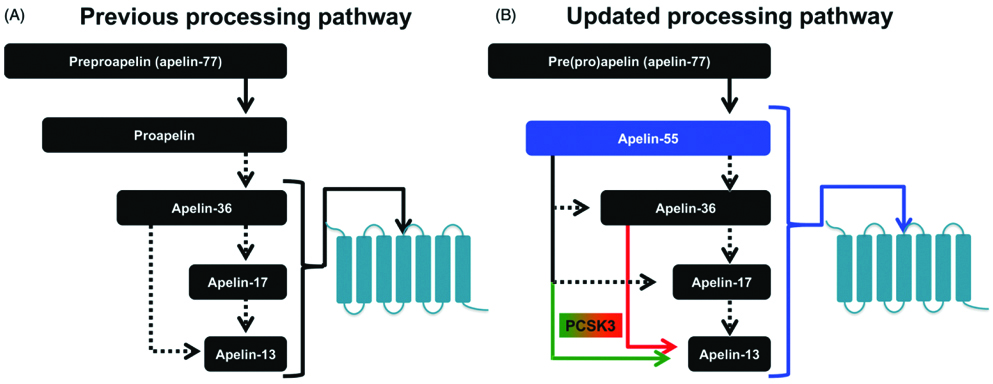
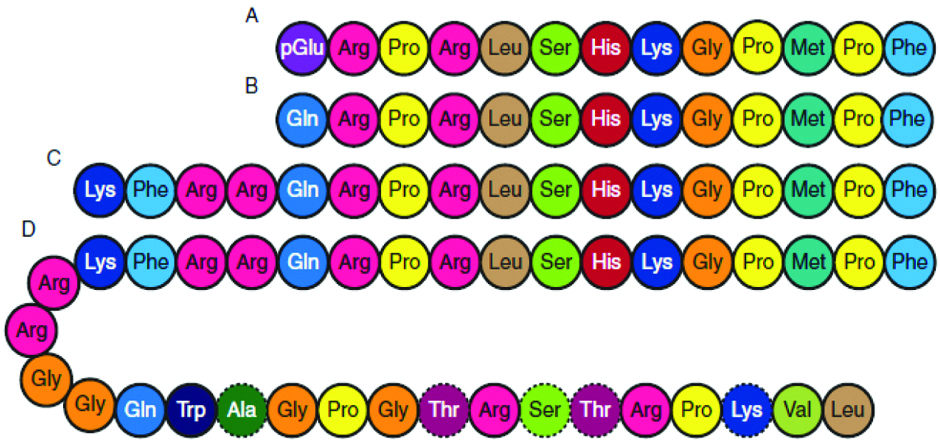
The term apelin denotes APJ endogenous ligand. APJ receptor remained as “orphan receptor” until 1998, when Tatemoto K et al., identified this ligand and termed it as apelin [2]. In humans, the APLN gene is located on chromosome X at Xq25-26.1 position; a 6 kb open reading frame is an apelin gene (APLN) with one intron. In human chromosome X, apelin gene (APLN) located at Xq25-26.1 and contains ~6 kb intron within its Open Reading Frame (ORF) [2]. Apelin is synthesised as 77 amino acid preproprotein (preproapelin), an immature single peptide, with hydrophobic rich N-terminal region. The amino acid sequence of apelin is similar to that of angiotensin II [2]. Bovine, human, rat and mouse preproapelin precursors have 76-95% homology and appear to exist endogenously as a dimeric protein, due to disulphide bridges. Dimerization of pre(pro)apelin occurs by disulphide bridge formation. Previous studies, reported that the dimerization is a prerequisite for proper processing in bioactive peptides, such as stomatostatin-II, suggesting that dimerization is necessary for proper processing of apelin [6,13]. In the endoplasmic reticulum, prepropeptide is cleaved to a 55 amino acid proapelin by removal of an N-terminal signal peptide, presumed to be an inactive precursor, containing the receptor binding site [14]. The 55 amino acids proapelin peptide further generate several smaller bioactive peptides, which includes apelin-36 (apelin 42-77), apelin-17 (apelin 61-77) and apelin-13 (apelin 65-77) [15]. Additionally, pyroglutamate apelin 13 {(Pyr1)-apelin 13} is a post-translationally modified form of apelin and it contains pyroglutamate group at N-terminus of the peptide [16]. These peptides have distinct activities and shorter peptides are more potent activators for APJ [Table/Fig-2,3] [17].
Apelin processing pathways. (a). Previously ‘theorised’ sequential processing pathway of “apelin-36 precursor” (b). Current ‘Myriad’ processing pathway based on recent findings of apelinergic system which identifies proapelin as apelin-55, an additional bioactive member [6].
Primary structure of apelin peptides and its amino acid sequences (A-Pyr1-apelin 13, B-Apelin 13, C-Apelin 17, D Apelin-36) [6].
In adipocytes, proprotein convertase subtilisin/kexin 3 (PCSK3 or furin) directly cleaves proapelin to apelin 13, with no production of longer isoforms [14]. Apelin peptides, which lack cysteine residues, are probably present in monomeric form. Apelin protein biological activity depends on carboxy terminal of the peptide comprising 12 amino acids [16]. Carboxy-terminal of preproapelin is rich in basic amino acids, which are potential cleavage sites during post-translational processing [2]. Among all apelin peptides, apelin-13 and (Pyr1) apelin-13 are the shortest, most prevalent and biologically active forms.
The processing of apelin precursor (prepropeptide) is complex. Aminopeptidases, serine proteases and to some extent carboxypeptidases, are responsible for the processing of apelin peptides. Angiotensin converting enzyme-2 (ACE-2) degrade apelin-13 and apelin-36 into inactive peptides by cleaving Pro-Phe bond and removes phenylalanine on C-terminus with high catalytic efficiency [16]. It has been suggested that (Pyr1) apelin-13 is more resistant to enzymatic cleavage by ACE-2. To date, ACE-2 is the only known enzyme for degradation of apelin. In the circulation, apelin is cleared very rapidly within a short plasma half-life of no longer than 8 minutes [18].
Regulation of Apelin (Apln) Gene
The Apelin gene expression is regulated by various transcription factors. A single-nucleotide polymorphism (SNP) study reported Sp1 role in the regulation of apelin gene expression [8]. The expression of Apelin gene is enhanced by tumour necrosis factor-α (TNF-α), via phosphoinositide-3 kinase (PI3K), c-Jun N-terminal kinase (JNK) and MEK1/2 (mitogen activated protein kinase 1/2) in adipocytes [19]. Apelin core promoter sequences in rat and humans contain putative binding sites for Upstream Stimulatory Factor (USF) 1/2, and overexpression of USF up-regulates apelin transcription [20,21].
Hormone Response Elements (HREs) are present in promoter and intron sequence of apelin gene in various species that causes up-regulation of apelin expression [Table/Fig-4]. In hypoxic conditions, apelin expression is up-regulated in cardiac myocytes wherein Hypoxia Inducible Factor-1α (HIF-1α) binds to HRE (-813/-826) located within the first intron of human Apelin gene and increases apelin expression in vascular cells [22]. In white adipocytes, peroxisome-proliferator-activated receptor γ co-activator 1α also up-regulates the Apelin gene expression [23].
Regulation of Apelin gene.
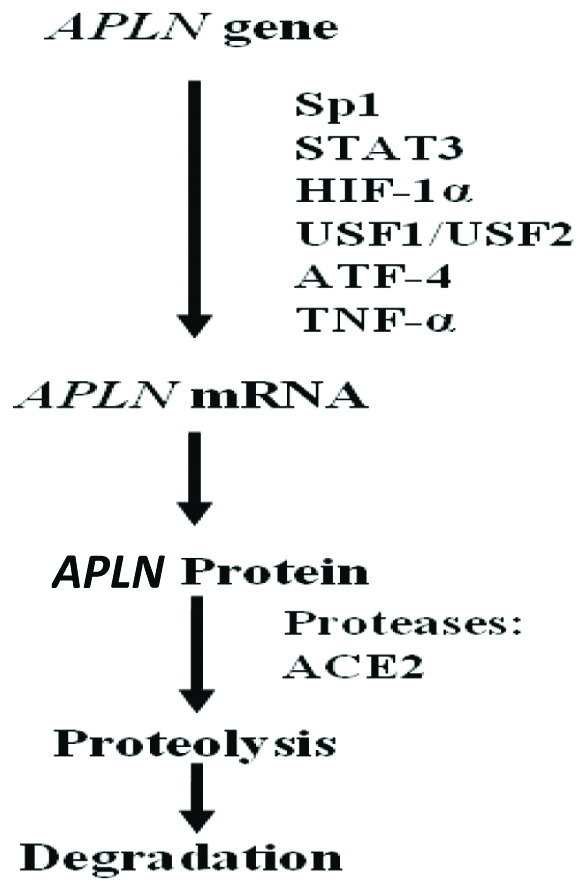
Sp1-Specificity protein 1; STAT3-Signal Transducer and Activator of Transcription 3; HIF-1α-Hypoxia Inducible Factor-1 α; USF1/USF2-Upstream Stimulatory Factor (USF) ½; ATF4-Activating Transcription Factor 4; TNF-α-Tumour Necrosis Factor α; ACE-II-Angiotensin Converting Enzyme-II [24].
Secretion and Distribution of Apelin and Apelin Receptor
In mammals, APJ expression is widely distributed in embryo and various peripheral tissues in adults. Maximum expression of APJ are found in the heart, lung, mammary gland and placenta, and significant but lower levels of APJ mRNA are present in kidney, pituitary gland, ovary and skeletal muscle, [25]. APJ expression in these tissues is localised to vascular, endothelial and smooth muscle cells. The apelin expression is also observed in a range of peripheral tissues, including heart, liver, kidney, adipose tissues, and brain, with highest levels found in the lung, mammary gland and placenta [26].
Receptor Binding and Mechanism of Action
Apelin is believed to be the only endogenous ligand for APJ [27]. All apelin peptides bind to APJ [14]. Binding of apelin peptides leads to conformational changes in the receptor, which causes activation of its associated G-protein that induces Guanosine Diphosphate (GDP) dissociation and Guanosine Triphosphate (GTP) binding. N-terminal domain of APJ plays a critical role in ligand binding [15]. APJ is suggested to couple with Gi/o protein, this findings was supported by the inability of (Pyr1) apelin 13 and apelin 36 to generate calcium mobilisation or to release arachidonic acid metabolites into the cells which leads to the positive or negative regulation of various intracellular effectors. By using transfected cells, similar results were obtained with neurons differentiated from NT2 (NTERA-2) cells and to a lesser extent with astrocytes. Interestingly, the rate of receptor recycling to the surface is faster for apelin 13 than for apelin 36 [28].
Cellular Effects and Intracellular Responses
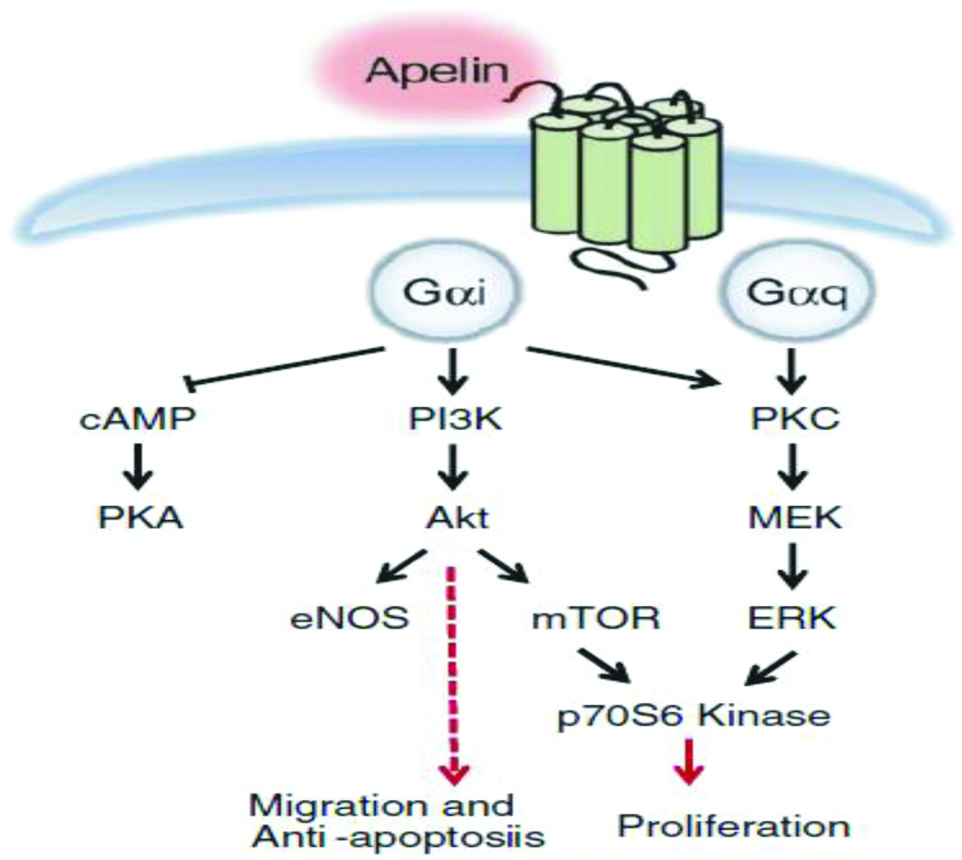
Apelin induces the proliferation and migration of APJ expressing endothelial cells [29]. The major signaling pathways of apelin are mediated initially by Gαi (inhibitory)-protein coupled to APJ and Protein Kinase C (PKC) [Table/Fig-5]. Apelin causes concentration-dependent inhibition of cAMP production and increases phosphorylation of protein kinase B in endothelial cells of umbilical cord. The binding of apelin to APJ, phosphatidylinositol-3 kinase (PI3K) and ERK pathway leads to phosphorylation of P70S6K, that results in endothelial cell migration and proliferation [30,31]. Studies have reported that APJ forms a heterodimer with k-opioid receptors and leads to ERK phosphorylation, causing increased cell proliferation [32]. Furthermore, apelin inhibits pulmonary arterial endothelial cell apoptosis in mouse [33]. The apelin-36 and apelin-13 induce intracellular effectors activation and exhibit varied coupling and desensitization through Gαi-protein [34]. Substitution of the C-terminal phenylalanine residue of apelin13 by alanine results in the generation of an antagonist of APJ. The only available nonpeptidic antagonist of APJ is the compound ALX40-4C (N-alpha-acetyl-nona-D-arginine amide acetate), which was primarily shown to interact with second extracellular loop of the CXCR4 (C-X-C motif Chemokine Receptor 4). However, it should be noted that binding affinity of ALX40-4C for CXCR4 is much higher than APJ receptor and thus it is not a specific antagonist to APJ [28].
Schematic representation of intracellular signal transduction pathway and cellular effects in apelin/APJ system [6].
cAMP-Cyclic adenosine monophosphate; PKA-Protein Kinase A; PI3K-Phosphatidylinositol 3-Kinase; Akt-Protein Kinase B; eNOS-Endothelial Nitric Oxide Synthase; mTOR-Mechanistic Target of Rapamycin; PKC-Protein Kinase C; ERK-Extracellular Regulated Kinase; MEK-Mitogen-activated Protein/Extracellular Signal-regulated Kinase
Quantification of Apelin
Human apelin is estimated in serum, plasma and other biological fluids by enzyme-linked immunosorbent assay (ELISA) and liquid chromatography/tandem mass spectrometry [35,36].
Biological Functions of Apelin
Apelin and APJ are distributed throughout the human body. Higher concentration of plasma apelin as compare to tissue concentration, suggests its paracine and autocrine nature.
Apelin and Cardiovascular Functions
The well-established apelin and its receptor APJ’s signaling pathways are associated with various cardiac and vascular functions. Apelin expression is primarily linked to the endothelium and APJ expression to the myocardium, suggesting a paracrine mode of signaling [37]. Apelin acts as a potent cardiac contraction activator; the apelin-APJ activation controls systemic perfusion and effect both heart and the vascular system [38]. In vascular system, Nitric Oxide (NO), mediates the effect of apelin as a vasodilator. In anaesthetised intact rats, peripherally administered apelin reduced the Mean Arterial Pressure (MAP) [39]. The effect of apelin and the specific apelin peptide in conscious state of the animal remains unclear but numerous studies suggests that apelin plays a cardinal role in blood pressure regulation [40-42]. Peripheral administration of apelin causes vasodilation in human saphenous vein; in contrast it acts as a vasoconstrictor in vessels denuded of endothelium [42]. Central administration of (Pyr1) apelin-13 increases the MAP [43]. While intracerebroventricular (ICV) administrations of (Pyr1) apelin 13 has no effect on MAP or heart rate in anaesthetised rats, ICV administrations increases the MAP and heart rate in conscious rats [44].
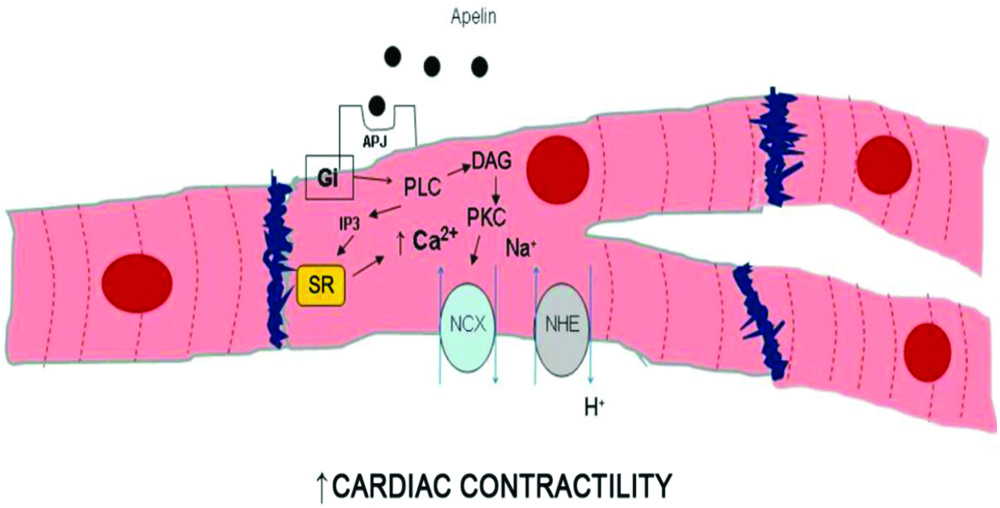
Apelin has been documented to have positive inotropic effects in normal rats and negative effect on failing heart rate of rats after myocardial infarction. Hence, it may be used as a positive inotropic agent in ischaemic heart failure patients. Apelin administration causes peripheral and coronary vasodilation and increases cardiac output. The inotropic effects of apelin may be caused by increased intracellular calcium levels mediated by Na+/H+ and Na+/Ca2+ exchanger activation [Table/Fig-6] [45].
Intracellular pathways responsible for positive inotropic effect of apelin/APJ interaction.
DAG-Diacylglycerol; Gi-Inhibitory G protein; IP3-Inositol Triphosphate; NCX-Na+/Ca2+ Exchanger; NHE-Na+/H+ Exchanger; PKC-Protein Kinase C; PLC-Phospholipase C; SR-Sarcoplasmic Reticulum [45]
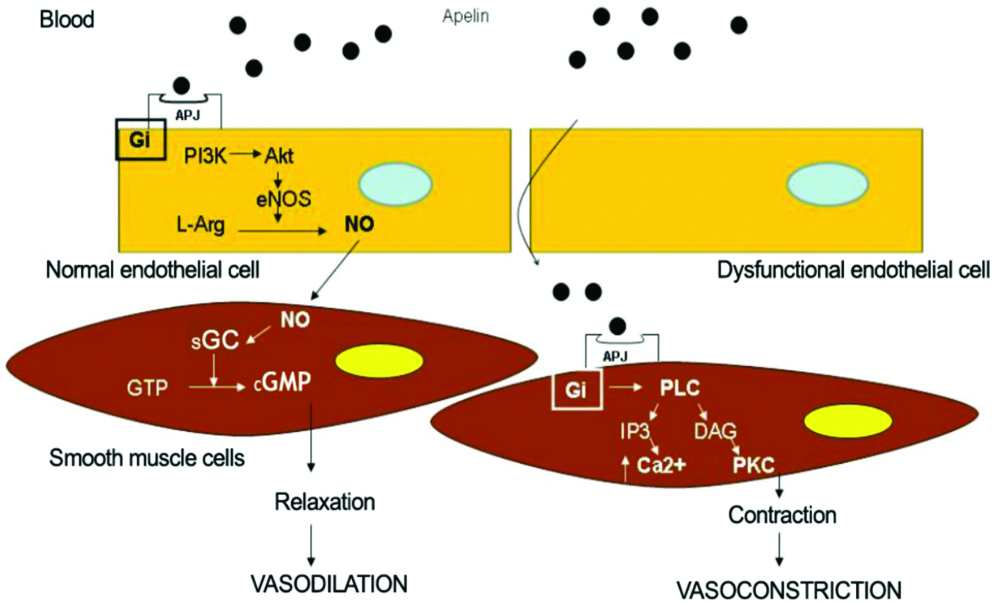
Studies have shown that apelin 13 administration causes reduction in the blood pressure in animal models and humans, which indicates that apelin can regulate blood pressure [13,43]. The ability of apelin’s blood pressure lowering effects differed depending on the dose, anaesthesia, route of administration and experimental model. Apelin causes endothelium dependent vasodilation by stimulating the phosphorylation of endothelial Nitric Oxide Synthase (eNOS) at serine 1179 and releases NO. Nitric oxide, in turn, activates soluble guanylate cyclase in vascular smooth muscle cells, resulting in increased levels of cyclic Guanosine Monophosphate (cGMP) which mediates dilation of vascular smooth muscle cells [15]. Apelin triggers L-arginine transport and increases the production of NO in isolated aorta of the rat [46]. Pyr1 apelin 13 also increases serum NO levels in infarcted treated rats [47]. Japp AG et al., reported that the use of apelin in humans stimulates peripheral vascular, coronary vasodilation and increases cardiac output [18]. However, administration of apelin in APJ knock-out mice indicated no change in blood pressure suggesting that apelin reduces blood pressure via its APJ receptor [48]. Apelin-induced endothelium-dependent vasodilation is reduced by co-administration of L-Nitro-Arginine-Methyl-Ester (L-NAME), suggesting an endothelium-derived NO dependent mechanism. L-NAME is an inhibitor of eNOS [Table/Fig-7] [16].
Intracellular pathways responsible for the vasomotor effects in apelin/APJ interaction in the absence and in the presence of endothelial dysfunction.
DAG-Diacylglycerol; eNOS-Endothelial Nitric Oxide Synthase; Gi-Inhibitory G protein; sGC-Soluble Guanylate Cyclase; GTP-Guanosine Triphosphate; cGMP-Cyclic Guanosine Monophosphate; IP3-Inositol Triphosphate; L-Arg-L-Arginine; NCE-Na+/Ca2+ Exchanger; NHE-Na+/H+ Exchanger; NO-Nitric Oxide; PKC-Protein Kinase C; PLC-Phospholipase C [45]
Role of Apelin in Vasoconstriction
Apelin increases the heart rate not directly through a chronotropic effect, but by a secondary baroreceptor reflex. An opposite effect was observed in conscious sheep; anesthetized and conscious rats, where apelin administration increased the heart rate and MAP [48]. Studies have shown that intravenous apelin-13 created a biphasic response in MAP and heart rate and this biphasic response consisted of a preliminary decrease, followed by a momentary increase and a decline back to the baseline level in the last 15 minutes. In vitro studies reported the apelin is involved in both vasodilation and vasoconstriction [49-51].
Role of Apelin in Angiogenesis
Apelin, angiogenic factor in endothelial cells, stimulates vessel growth and endothelial cell proliferation. In frogs, apelin is required for normal development of heart and blood vessels [52]. Additionally, development of retinal vasculature is decreased in apelin knock-out mice. Apelin is important for the hypoxia-induced retinal angiogenesis and is also involved in non-neovascular remodeling of the retina. Apelin activates the cell transduction cascade ERKs, Akt and p70S6 kinase phosphorylation, which leads to the proliferation of endothelial cells and formation of new blood vessels. These findings indicate that apelin is required for angiogenesis. However, further research is required to establish human model.
Role of Apelin in Fluid Homeostasis
APJ mRNA expression detection by Immunohistochemistry (IHC) in brain, shows that apelin regulates the body fluid homeostasis. Studies showed that both apelin 13 and apelin17 induce a decrease in circulating plasma vasopressin levels and water intake after ICV administration. Increased water consumption was observed in rats, upon intra-peritoneally administration of apelin. These contrasting effects can be explained on the account of different routes of administration [29]. In APJ knockout mice, abnormal fluid homeostasis leads to decrease in drinking behaviour and inability to concentrate, indicating apelin’s in vivo anti-diuretic effect [26].
Role of Apelin in Neuro-Endocrine Response to Stress
The expression of apelinergic system has been observed in the Central Nervous System (CNS). Existing scientific evidences confirm that the in vivo effects of apelin and APJ on Hypothalamic-Pituitary-Adrenal neuro-endocrinal functions are mediated through Corticotrophin Releasing Factor (CRF) and vasopressin dependent mechanisms.
Central administration of (Pyr1) apelin 13 increases the expression of c-fos, an indicator of neuronal activity in the paraventricular nucleus [40]. Furthermore, apelin 13 administration stimulates the release of Corticotrophin Releasing Hormone (CRH) and vasopressin from hypothalamic extracts in vitro. In the paraventricular nucleus, APJ mRNA levels increase in response to acute and chronic stress and following adrenalectomy, implying negative regulation of APJ mRNA expression by glucocorticoids [53].
Role of Apelin in Metabolic Actions
Apelin and its ligand APJ are expressed in adipose tissue of mouse, human and rats. Apelin controls food intake and a positive correlation have been reported between plasma apelin and Body Mass Index (BMI). Apelinergic system is a therapeutic target for metabolic disorders. However, expression of plasma apelin is increased only in obese subjects and in mouse models of obesity associated with hyperinsulinaemia, indicating that obesity or high fat diet feeding may not be the main cause for the rise in the apelin expression thus implies a close relationship between apelin and insulin both in vivo and in vitro [54].
Insulin stimulates the production of apelin in adipocytes and apelin mRNA expression is down-regulated in the adipocytes of mice treated with the β-cell toxin streptozotocin, which leads to a fall in plasma insulin levels [55]. Intraperitoneal apelin administration in normal and obese mice reduced body adiposity without affecting food intake, decreased the insulin, leptin and triglyceride levels and increased adiponectin levels. Apelin is necessary for insulin sensitivity. Reduced insulin sensitivity, glucose intolerance and hyperinsulinaemia are observed in apelin knockout mice [56]. Peripheral administrated apelin have powerful glucose lowering effect and it increased glucose uptake in skeletal muscle, adipose tissue and improved insulin sensitivity in both apelin knockout and obese high-fat diet fed mice [57].
Therapeutic Applications of Apelin
Studies suggest the therapeutic potential of apelin with respect to treatment of hypertension and Cardiovascular Diseases (CVD) in animal models, even though its short half-life limits the therapeutic uses of apelin. Based on these findings, the recent research is focused on the development of novel agonists, delivery methods, and/or improving the efficacy of agonists of APJ.
The role of apelin’s blood pressure lowering effects, by virtue of its C-terminal phenylalanine, plays an important role, evident by its substitution for alanine results in apelin-specific antagonistic activity in vivo and reduced apelin binding affinity in vitro [58]. Apelin treatment in rats with isoprenalin-induced lesions promotes the restoration of cardiac function and is also cardioprotective against lipidic peroxidation [59]. Ventricular resynchronisation therapy promotes a long-term increase in plasma levels of apelin, which may be associated to cardiac function improvement [60]. Reduced levels of apelin are reported in coronary disease patients undergoing haemodialysis. It is speculated that apelin may be involved in the pathophysiology of CVD, secondary to chronic renal failure and that it may be utilised in the future as a treatment approach for uraemic cardiomyopathy [61].
Apelin is considered a possible marker for cardiac hypoxia (acute and chronic). Circulating levels of apelin do not seem to be of help for the clinical evaluation and prognosis of acute or chronic heart failure patients [62]. Apelin may be a useful marker to assess the development of isolated atrial fibrillation as well as a diagnostic marker to distinguish dyspnoea due to pulmonary causes from that of cardiovascular causes.
Apelin gene therapy has also been proved to be cardioprotective by stimulating angiogenesis in diabetic mice or mice subjected to myocardial infarction [63]. As per the available information, the therapeutic potential of apelin in humans is scarce and draws much attention for researchers.
Recent Updates
Van Mieghem T et al., analysed maternal serum concentration of apelin and cardiac output and total peripheral resistance in high risk pregnancies at 20 to 34 weeks of gestation. Placental apelin staining was done by IHC and placental apelin gene expression was assessed by quantitative polymerase chain reaction (qPCR). In 20 and 26 weeks of gestation, apelin levels were found to be positively correlated with total peripheral resistance (r=.57, p=0.01) and inversely correlated with stroke volume (r=-42, p=0.08). About 30% reduction in apelin serum levels was observed in pregnancies with Intrauterine Growth Restriction (IUGR) complications as compared to uncomplicated pregnancies or in women with preeclampsia (p=0.009). Apelin staining was strongly decreased in IUGR compared to controls. Preeclamptic placentas showed an intermediate staining. Serum and placental apelin were significantly reduced in IUGR condition and apelin levels mimicked cardiovascular changes during pregnancy [64].
Colcimen N et al., carried out a study on the role of Vascular Endothelial Growth Factor (VEGF), annexin A5 and apelin by IHC in placenta of preeclamptic and healthy controls. Between placenta samples of normotensive pregnant (controls), mild preeclampsia and severe preeclampsia cases. VEGF, annexin A5 and apelin were measured by immunohistochemical methods and Immunoreactivity (IRS) scores were calculated for each parameter. Results of the above study displayed increased levels of VEGF and apelin IRS in mild and severe preeclamptic group (p<0.026) in response to control group (p<0.002). However, annexin A5 IRS was significantly decreased in preeclampsia (p<0.04). Depending upon the intensity of the disease, it can be stated that elevated VEGF and apelin and low level of annexin A5 are responsible for alteration in fetoplacental circulation and structure in uteroplacental bed in preeclampsia [65].
Yamaleyeva LM et al., studied the expression pattern of apelin content in chorionic villi of normal pregnant and preeclamptic women at gestational age of 36-38 weeks. In preeclampsia, the preproapelin mRNA levels are reduced, which was associated with low total apelin content in preeclamptic women as compared with normal chorionic villi. Further, apelin peptide was characterised in pooled samples of each group by using HPLC-RIA, which revealed little or no apelin 36 or apelin 17. Pyroglutamate apelin 13 {(Pyr1)-apelin 13} was the predominant form in preeclamptic and normal villi. The activity of ACE-2 was higher in preeclamptic villi and decreased apelin levels were observed in placental chorionic villi of preeclamptic women [16].
Therefore, the conclusion is that angiotensin II involving regulation of apelin expression which is decreased in preeclamptic villi.
Future Prospective
Apelin may serve as a novel drug target linked to cardiovascular disease, hypertension and preeclampsia. The intensity of stress level alters the growth and development while contributing to physiological and metabolic changes. From the present knowledge, there is a possibility that apelin/APJ alters this balance. Acute stress triggers immunological reactions, alterations in blood pressure, GI symptoms, neurological and psychological responses while chronic stress causes behavioural and neuropsychiatric manifestations, cardiovascular and metabolic diseases. The apelinergic system has been implicated in most of these conditions. Hence, targeting apelin may bring new therapeutic options to overcome all cardiovascular related problems in the near future.
Conclusion
Apelin peptides and its receptor APJ are widely expressed in vivo, with interactions between apelin peptides and APJ. These participate in the regulation of key roles in cardiovascular system, angiogenesis, fluid homeostasis, neuro-endocrine response to stress, metabolic actions, therapeutic role in vasodilation and constriction. This article suggests the need for further augmentation of research evidences in human system with respect to various biological functions.
[1]. O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, A Human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11Gene 1993 136(1-2):355-60.10.1016/0378-1119(93)90495-O [Google Scholar] [CrossRef]
[2]. Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptorBiochem Biophys Res Commun 1998 251(2):471-76.10.1006/bbrc.1998.94899792798 [Google Scholar] [CrossRef] [PubMed]
[3]. Ostrom RS, Insel PA, The Evolving role of lipid rafts and caveolae in G protein coupled receptor signaling: Implications for molecular pharmacologyBr J Pharmacol 2004 143(2):235-45.10.1038/sj.bjp.070593015289291 [Google Scholar] [CrossRef] [PubMed]
[4]. Wheatley M, Hawtin SR, Glycosylation of G-protein coupled receptors for hormones central to normal reproductive functioning: its occurrence and roleHum Reprod Update 1999 5(4):356-64.10.1093/humupd/5.4.35610465525 [Google Scholar] [CrossRef] [PubMed]
[5]. Zhou N, Fan X, Mukhtar M, Fang J, Paterl CA, Du Bois GC, Cell-cell fusion and internalization of the CNS-based, HIV-1 co-receptor APJVirology 2003 307(1):22-36.10.1016/S0042-6822(02)00021-1 [Google Scholar] [CrossRef]
[6]. Shin K, Kenward C, Rainey JK, Apelinergic system structure and functionCompr Physiol 2017 8(1):407-50.10.1002/cphy.c17002829357134 [Google Scholar] [CrossRef] [PubMed]
[7]. O’Carroll AM, Lolait SJ, Howell GM, Transcriptional regulation of the rat apelin receptor gene: Promotor cloning and identification of an Sp1 site necessary for promoter activityJ Mol Endocrinol 2006 36(1):221-35.10.1677/jme.1.0192716461940 [Google Scholar] [CrossRef] [PubMed]
[8]. Hata J, Matsuda K, Ninomiya T, Yonemoto K, Matsushita T, Ohnishi Y, Functional SNP in an Sp1-binding site of AGTL1 gene is associated with susceptibility to brain infarctionHum Mol Genet 2007 16(8):630-39.10.1093/hmg/ddm00517309882 [Google Scholar] [CrossRef] [PubMed]
[9]. Sarzani R, Forleo C, Pietrucci F, Capestro A, Soura E, Guida P, The 212A variant of the APJ receptor gene for the endogenous inotrope apelin is associated with slower heart failure progression in idiopathic dilated cardiomyopathyJ Card Fail 2007 13(7):521-29.10.1016/j.cardfail.2007.04.00217826642 [Google Scholar] [CrossRef] [PubMed]
[10]. Falcone C, Bozzini S, Schirinzi S, Buzzi MP, Boiocchi C, Totaro R, APJ polymorphisms in coronary artery disease patients with and without hypertensionMol Med Rep 2012 5(2):321-25. [Google Scholar]
[11]. O’Carroll AM, Don AL, Lolait SJ, APJ receptor mRNA expression in the rat hypothalamic paraventricular nucleus: regulation by stress and glucocorticoidsJ Neuroendocrinol 2003 15(11):1095-101.10.1046/j.1365-2826.2003.01102.x14622440 [Google Scholar] [CrossRef] [PubMed]
[12]. Dray C, Debard C, Jager J, Disse E, Daviaud D, Martin P, Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humansAm J Physiol Endocrinol Metab 2010 298(6):E1161-69.10.1152/ajpendo.00598.200920233941 [Google Scholar] [CrossRef] [PubMed]
[13]. Lee DK, Saldivia VR, Nguyen T, Cheng R, George SR, O’Dowd BF, Modification of the terminal residue of apelin 13 antagonizes its hypotensive actionEndocrinology 2005 146(1):231-36.10.1210/en.2004-035915486224 [Google Scholar] [CrossRef] [PubMed]
[14]. Shin K, Pandey A, Liu XQ, Anini Y, Rainey JK, Preferential apelin 13 production by the proproteinconvertase PCSK3 is implicated in obesityFEBS Open Bio 2013 3:328-33.10.1016/j.fob.2013.08.00124251091 [Google Scholar] [CrossRef] [PubMed]
[15]. Ibrahim NA, The impact of apelin ligand binding on APJ receptor on the various physiological and pathophysiological aspects of metabolism and homeostasisEur J Pharm Med Res 2017 4(1):61-69. [Google Scholar]
[16]. Yamaleyeva LM, Chappell M, Brosnihan KB, Anton L, Caudell DL, Shi S, Down-regulation of apelin in the human placental chorionic villi from preeclamptic pregnanciesAm J Physiol Endocrinol Metab 2015 309(10):E852-60.10.1152/ajpendo.00272.201526394665 [Google Scholar] [CrossRef] [PubMed]
[17]. Kidoya H, Takakura N, Biology of the apelin-APJ axis in vascular formationJ Biochem 2012 152(2):125-31.10.1093/jb/mvs07122745157 [Google Scholar] [CrossRef] [PubMed]
[18]. Japp AG, Cruden NL, Amer DA, Li VK, Goudie EB, Johnston NR, Vascular effects of apelin in vivo in manJ Am Coll Cardiol 2008 52(11):908-13.10.1016/j.jacc.2008.06.01318772060 [Google Scholar] [CrossRef] [PubMed]
[19]. Daviaud D, Boucher J, Gesta S, Dray C, Guigne C, Quilliot D, TNF-α up-regulates apelin expression in human and mouse adipose tissueFASEB J 2006 20(9):1528-30.10.1096/fj.05-5243fje16723381 [Google Scholar] [CrossRef] [PubMed]
[20]. Wang G, Qi X, Wei W, Ella W, Englander EW, Greeley GH Jr, Characterization of the 5’-regulatory regions of the rat and human apelin genes and regulation of breast apelin by USFFASEB J 2006 20:2639-41.10.1096/fj.06-6315fje17060400 [Google Scholar] [CrossRef] [PubMed]
[21]. Han S, Wang G, Qi X, Lee HM, Englander EW, Greely GH Jr, A possible role for hypoxia-induced apelin expression in enteric cell proliferationAm J Physiol Regul Integr Comp Physiol 2008 294(6):R1832-39.10.1152/ajpregu.00083.200818367654 [Google Scholar] [CrossRef] [PubMed]
[22]. Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesisCirc Res 2008 103(4):432-40.10.1161/CIRCRESAHA.108.17933318617693 [Google Scholar] [CrossRef] [PubMed]
[23]. Mazzucotelli A, Ribet C, Castan-Laurell I, Daviaud D, Guigne C, Langin D, The transcriptional co-activator PGC-1α up regulates apelin in human and mouse adipocytesRegul Pept 2008 150(1-3):33-37.10.1016/j.regpep.2008.04.00318501443 [Google Scholar] [CrossRef] [PubMed]
[24]. Wu Y, Wang X, Zhou X, Cheng B, Li G, Bai B, Temporal expression of Apelin/Apelin receptor in ischemic stroke and its therapeutic potentialFront Mol Neurosci 2017 10:1-8.10.3389/fnmol.2017.00001 [Google Scholar] [CrossRef]
[25]. Kleinz MJ, Devenport AP, Emerging roles of apelin in biology and medicinePharmacol Ther 2005 107(2):198-11.10.1016/j.pharmthera.2005.04.00115907343 [Google Scholar] [CrossRef] [PubMed]
[26]. Roberts EM, Pope GR, Newson MJ, Landgraf R, Lolait SJ, O’Carroll AM, Stimulus-specific neuroendocrine responses to osmotic challenges in apelin receptor knockout miceJ Neuroendocrinol 2010 22(4):301-08.10.1111/j.1365-2826.2010.01968.x20136689 [Google Scholar] [CrossRef] [PubMed]
[27]. Andersen CU, Hilberg O, Mellemkjcer S, Nielsen-Kudsk JE, Simonsen U, Apelin and pulmonary hypertensionPulm Circ 2011 1(3):334-46.10.4103/2045-8932.8729922140623 [Google Scholar] [CrossRef] [PubMed]
[28]. Apelin Receptor, UCSD-Nature molecule pagesPublished online: 7 Apr 2006 http://www.signaling-gateway.org/molecule/query?afcsid=A000304 [Google Scholar]
[29]. Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, Apelin is a novel angiogenic factor in retinal endothelial cellsBiochem Biophys Res Commun 2004 325(2):395-400.10.1016/j.bbrc.2004.10.04215530405 [Google Scholar] [CrossRef] [PubMed]
[30]. Masri B, Lahlou H, Mazarguil H, Knibiehler B, Audigier Y, Apelin (65-77) activates extracellular signal-regulated kinases via a PTX-sensitive G proteinBiochem Biophys Res Commun 2002 290(1):539-45.10.1006/bbrc.2001.623011779205 [Google Scholar] [CrossRef] [PubMed]
[31]. Hashimoto Y, Ishida J, Yamamoto R, Fujiwara K, Asada S, Kasuya Y, G protein-coupled APJ receptor signaling induces focal adhesion formation and cell motilityInt J Mol Med 2005 16(5):787-92.10.3892/ijmm.16.5.787 [Google Scholar] [CrossRef]
[32]. Li Y, Chen J, Bai B, Du H, Liu Y, Liu H, Heterodimerization of human apelin and kappa opioid receptors: Roles in signal transductionCell Signal 2012 24(5):991-1001.10.1016/j.cellsig.2011.12.01222200678 [Google Scholar] [CrossRef] [PubMed]
[33]. Xie H, Yuan LQ, Luo XH, Huang J, Cui RR, Guo LJ, Apelin suppresses apoptosis of human osteoblastsApoptosis 2007 12(1):247-54.10.1007/s10495-006-0489-717136493 [Google Scholar] [CrossRef] [PubMed]
[34]. Masri B, Morin N, Pedebernade L, Knibiehler B, Audigier Y, Theapelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragmentsJ Biol Chem 2006 281(27):18317-26.10.1074/jbc.M60060620016679320 [Google Scholar] [CrossRef] [PubMed]
[35]. Porstmann T, Kiessig ST, Enzyme immunoassay techniques. An overviewJ Immunol Methods 1992 150(1-2):5-21.10.1016/0022-1759(92)90061-W [Google Scholar] [CrossRef]
[36]. Mesmin C, Dubois M, Becher F, Fenaille F, Ezan E, Liquid chromatography/tandem mass spectrometry assay for the absolute quantification of the expected circulating apelin peptides in human plasmaRapid Commun Mass Spectrom 2010 24(19):2875-84.10.1002/rcm.471820857448 [Google Scholar] [CrossRef] [PubMed]
[37]. Charo DN, Ho M, Fajardo G, Kawana M, Kundu RK, Sheikh AY, Endogenous regulation of cardiovascular function by apelin-APJAm J Physiol Heart Circ Physiol 2009 297(5):H1904-H1913.10.1152/ajpheart.00686.200919767528 [Google Scholar] [CrossRef] [PubMed]
[38]. Szokodi I, Tavi P, Foldes G, Voutilainen-Myllyla S, Ilves M, Tokola H, Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractilityCirc Res 2002 91:434-40.10.1161/01.RES.0000033522.37861.6912215493 [Google Scholar] [CrossRef] [PubMed]
[39]. Reaux A, De Mota N, Skultetyova I, Lenkei Z, EI Messari S, Gallatz K, Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brainJ Neurochem 2001 77(4):1085-96.10.1046/j.1471-4159.2001.00320.x11359874 [Google Scholar] [CrossRef] [PubMed]
[40]. Kagiyama S, Fukuhara M, Matsumura K, Lin Y, Fujii K, Lida M, Central and peripheral cardiovascular actions of apelin in conscious ratsRegul Pept 2005 125(1-3):55-59.10.1016/j.regpep.2004.07.03315582714 [Google Scholar] [CrossRef] [PubMed]
[41]. Ishida J, Hashimoto Y, Hashimoto T, Nishiwaki S, Iguchi T, Harada S, Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivoJ Biol Chem 2004 279(25):26274-79.10.1074/jbc.M40414920015087458 [Google Scholar] [CrossRef] [PubMed]
[42]. Katugampola SD, Maguire JJ, Matthewson SR, Davenport AP, [(125)I]-(Pyr(1)) apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in manBr J Pharmacol 2001 132(6):1255-60.10.1038/sj.bjp.070393911250876 [Google Scholar] [CrossRef] [PubMed]
[43]. Seyedabadi M, Goodchild AK, Pilowsky PM, Site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respirationAuton Neurosci 2002 101(1-2):32-38.10.1016/S1566-0702(02)00178-9 [Google Scholar] [CrossRef]
[44]. Mitra A, Kotovich MJ, Mecca A, Rowland NE, Effects of central and peripheral injections of apelin on fluid intake and cardiovascular parameters in ratsPhysiol Behav 2006 89(2):221-25.10.1016/j.physbeh.2006.06.00616839572 [Google Scholar] [CrossRef] [PubMed]
[45]. Ladeiras-Lopes R, Ferreira-Martins J, Leite-Moreira AF, The apelinergic system: the role played in human physiology and pathology and potential therapeutic applicationsArq Bras Cardiol 2008 90(5):343-49.10.1590/S0066-782X200800050001218516406 [Google Scholar] [CrossRef] [PubMed]
[46]. Jia YX, Lu ZF, Zhang J, Pan CS, Yang JH, Zhao J, Apelin activates L-arginine/nitric oxide synthase/nitric oxide pathway in rat aortasPeptides 2007 28(10):2023-29.10.1016/j.peptides.2007.07.01617719140 [Google Scholar] [CrossRef] [PubMed]
[47]. Azizi Y, Faghihi M, Imani A, Roghani M, Nazari A, Post-infarct treatment with [Pyr1] apelin 13 reduces myocardial damage through the reduction of oxidative injury and nitric oxide enhancement in the rat model myocardial infarctionPeptides 2013 46:76-82.10.1016/j.peptides.2013.05.00623727032 [Google Scholar] [CrossRef] [PubMed]
[48]. Japp AG, Cruden NL, Barnes G, van Gemeren N, Mathews J, Adamson J, Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failureCirculation 2010 121(16):1818-27.10.1161/CIRCULATIONAHA.109.91133920385929 [Google Scholar] [CrossRef] [PubMed]
[49]. Charles CJ, Rademaker MT, Richards AM, Apelin 13 induces a biphasic haemodynamic response and hormonal activation in normal conscious sheepJ Endocrinol 2006 189(3):701-10.10.1677/joe.1.0680416731800 [Google Scholar] [CrossRef] [PubMed]
[50]. Pitkin SL, Maguire JJ, Bonner TI, Davenport AP, International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and functionPharmacol Rev 2010 62(3):331-42.10.1124/pr.110.00294920605969 [Google Scholar] [CrossRef] [PubMed]
[51]. Castan-Laurell I, Dray C, Attane C, Duparc T, Knauf C, Valet P, Apelin, diabetes, and obesityEndocrine 2011 40(1):1-9.10.1007/s12020-011-9507-921725702 [Google Scholar] [CrossRef] [PubMed]
[52]. Inui M, Fukui A, Ito Y, Asashima M, Xapelin and Xmsr are required for cardiovascular development in Xenopus laevisDev Biol 2006 298(1):188-200.10.1016/j.ydbio.2006.06.02816876154 [Google Scholar] [CrossRef] [PubMed]
[53]. O’Carroll AM, Lolait SJ, Regulation of rat APJ receptor messenger ribonucleic acid expression in magnocellular neurons of the paraventricular and supraoptic nuclei by osmotic stimuliJ Neuroendocrinol 2003 15:661-66.10.1046/j.1365-2826.2003.01044.x12787050 [Google Scholar] [CrossRef] [PubMed]
[54]. Castan-Laurell I, Vitkova M, Daviaud D, Dray C, Kovacikova M, Kovacova Z, Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJEur J Endocrinol 2008 158(6):905-10.10.1530/EJE-08-003918390990 [Google Scholar] [CrossRef] [PubMed]
[55]. Sorhede Winzell M, Magnusson C, Ahren B, The APJ receptor is expressed in pancreas islets and its ligand, apelin, inhibits insulin secretion in miceRegul Pept 2005 131(1-3):12-17.10.1016/j.regpep.2005.05.00415970338 [Google Scholar] [CrossRef] [PubMed]
[56]. Yue P, Jin H, Aillaud M, Deng AC, Azuma J, Asagami T, Apelin is necessary for the maintenance of insulin sensitivityAm J Physiol Endocrinol Metab 2010 298(1):E59-E67.10.1152/ajpendo.00385.200919861585 [Google Scholar] [CrossRef] [PubMed]
[57]. Attane C, Foussal C, Le Gonidec S, Benani A, Daviaud D, Wanecq E, Apelin treatment increases complete fatty acid oxidation, mitochondrial oxidative capacity and biogenesis in muscle of insulin-resistant miceDiabetes 2012 61(2):310-20.10.2337/db11-010022210322 [Google Scholar] [CrossRef] [PubMed]
[58]. Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelinJ Neurochem 2003 84(5):1162-72.10.1046/j.1471-4159.2003.01587.x12603839 [Google Scholar] [CrossRef] [PubMed]
[59]. Jia YX, Pan CS, Zhang J, Geng B, Zhao J, Gerns H, Apelin protects myocardial injury induced by isoproterenol in ratsRegul Pept 2006 133(1-3):147-54.10.1016/j.regpep.2005.09.03316278022 [Google Scholar] [CrossRef] [PubMed]
[60]. Francia P, Salvati A, Balla C, De Paolis P, Pagannone E, Borro M, Cardiac resynchronization therapy increases plasma levels of the endogenous inotrope apelinEur J Heart Fail 2007 9(3):306-09.10.1016/j.ejheart.2006.06.00516891152 [Google Scholar] [CrossRef] [PubMed]
[61]. Malyszko J, Malyszko JS, Hryszko T, Mysliwiec M, Thrombin activatable fibrinolysis inhibitor in hypertensive kidney transplant recipientsTransplant Proc 2006 38(1):105-07.10.1016/j.transproceed.2005.11.07216504676 [Google Scholar] [CrossRef] [PubMed]
[62]. Van Kimmenade RR, Januzzi JL Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failureJ Am Coll Cardiol 2006 48(6):1217-24.10.1016/j.jacc.2006.03.06116979009 [Google Scholar] [CrossRef] [PubMed]
[63]. Azizi Y, Faghihi M, Imani A, Roghani M, Zekri A, Mobasheri MB, Post-infarct treatment with [Pyr(1)]apelin-13 improves myocardial function by increasing neovascularization and overexpression of angiogenic growth factors in ratsEur J Pharmacol 2015 761:101-08.10.1016/j.ejphar.2015.04.03425936512 [Google Scholar] [CrossRef] [PubMed]
[64]. Van Mieghem T, Doherty A, Baczyk D, Drewlo S, Baud D, Carvalho J, Apelin in normal pregnancy and pregnancies complicated by placental insufficiencyReprod Sci 2016 23(8):1037-43.10.1177/193371911663042226880769 [Google Scholar] [CrossRef] [PubMed]
[65]. Colcimen N, Bulut G, Ergul Erkec O, Ragbetli MC, Investigation of role of vascular endothelial growth factor, Annexin A5 and Apelin by immunohistochemistry method in placenta of preeclampsia patientsCell Mol Biol (Noisy-le-grand) 2017 63(11):42-45.10.14715/cmb/2017.63.11.829208172 [Google Scholar] [CrossRef] [PubMed]