RA is a systemic autoimmune disease which affects 0.5-1% of the general population [1,2]. It is a chronic disability leading to 70% irreversible joint damage which can be prevented if diagnosed and treated early [1-4]. There is no single gold standard clinical, pathologic, laboratory or radiological feature for diagnosis of RA [5]. Factors responsible for the development of RA are unclear and unpredictable, rendering diagnosis difficult [6,7].
Early diagnosis and prompt treatment with Disease Modifying Anti Rheumatic Drugs (DMARD) interfere with the disease progression thereby preventing the deformities and improving quality of life of the patient [8,9]. The present diagnostic criteria for RA are based on American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) [5]. Apart from clinical presentations the ACR/EULAR classification criteria includes RF and Anti-CCP Ab as serological markers [1].
The RF factor has acceptable sensitivity and low specificity as it is present even in elderly persons or in patients with connective tissue disorders and other autoimmune diseases [1,10]. ACR recommends RF factor as a screening test in diagnosis of RA, which has a sensitivity of 80% [10].
Recent data suggest that IgM-RF plays an important role in pathogenesis of RA. In addition, many studies have highlighted Anti-cyclic citrullinated Ab assay as a serological diagnostic tool even in RF negative patients RA has been emerging [10]. The discrimination between RA and non RA patients are better diagnosed using Anti-CCP [10,11]. This provides early diagnosis and better prognosis of RA by assessing the Anti-CCP Ab [5,7,12]. Since only few studies are available to demonstrate the application of Anti-CCP as serological marker for the diagnosis of RA the present study was carried out to compare Anti-CCP ELISA, RF IgM ELISA and RF latex agglutination tests to observe the combined sensitivity and specificity of the tests in early diagnosis of RA.
Materials and Methods
The study was carried out after obtaining Institutional Human Ethics Committee approval (72/IHEC/9-16). This cross-sectional descriptive study was conducted at the Department of Microbiology, Chettinad Hospital and Research Institute, Kelambakkam, Chennai, Tamil Nadu, India (November 2016 to May 2018).
Blood samples were collected after obtaining the informed written consent from 60 patients with complaints of polyarthropathy (General Medicine and Orthopaedics department).
The study included adult patients above 18 years of age, comprising both males and females, with symmetrical poyarthropathy, fulfilling the ACR criteria for RA along with family history of RA. Patients already diagnosed to have RA and on treatment were excluded.
The study tool consisted of questionnaires which contained details on socio demographical features such as age, sex, socioeconomic status and family history of RA.
Serum was separated from four mL of venous blood and was collected aseptically in BD (Beckton Dickinson) Serum Activator Tube. Serum was separated after centrifuging the sample at 4000 revolutions per minute (rpm) for five minutes. The separated serum was stored at -20°C in a sterile Eppendoff tubes.
RF determination by latex agglutination test was performed using Ensure Biotech Kit, which detects RF IgM. The test was performed as per the kit instructions of Ensure Biotech [13]. The antigen for the RF Latex Agglutination test is synthesised from polystyrene latex particles which are coated with a special preparation of purified human Gamma globulin. On mixing the latex particles with a sample which contains RF, agglutination occurs in the mixture and this is interpreted as positive. (Latex coated with IgM+serum containing RF=IgM Antibody binds with IgM and cause latex particle to agglutinate). Agglutination depends on concentration of RF in serum that may be equal or greater than sensitivity mentioned as detectable by the slide test method.
Both Qualitative method and Semi quantitative method were performed according to the manufacturer’s instruction. In Qualitative method, distinct agglutination indicates RF content >12 IU RF/mL in undiluted serum. In semi quantitative method, the highest dilution of serum giving agglutination within two minutes corresponded to the RA titer. The concentration of RA was calculated as follows:
RA in IU/mL=D×S, where D is the highest dilution showing clear cut agglutination and S is the sensitivity of the test: 12 IU/mL
RF IgM Antibody detection by enzyme immunoassay was performed using the Euroimmun kit, according to manufacturer’s instruction [14]. In short, the test kit containing microtiter strips each with eight break-off reagent wells coated with IgG was brought to room temperature. After the required number of wells was broken off, the patient samples were added to the designated microwells and incubated. If the patient serum contained RF, they will bind to the antigens coated in the microwells. In the next step following a wash procedure, enzyme labeled anti-human IgM conjugate was added. Then a substrate was added followed by a stop solution [14]. Then the absorbance was measured using an ELISA reader. Samples were interpreted as positive when their absorbance was more than the Optical Density (OD) of the calibrator corresponding to 20 RU/mL and negative when their OD was less than that of the calibrator.
IgG Anti-CCP antibody detection by enzyme immunoassay was performed by using the Autobind ELISA kit [15]. The microwells contained bound Cyclic Citrullinated Peptides. If the patient’s serum contained Anti-CCP antibodies, they will bind to the antigen. The wells were then washed to remove nonspecific proteins. Then enzyme conjugated with antibodies to Anti-CCP was added and incubated. This will bind to the patient’s antibody which has bound to the antigen. This step was followed by the addition of a substrate and a stop solution. The OD values of the controls and samples were measured at 450 nm.
OD was directly proportional to the concentration of citrullinated protein antibodies in the sample. Samples with OD greater than 20 U/mL- Standard were considered as positive. Samples with OD less 20 U/mL- Standard were considered to be negative.
Statistical Analysis
Statistical analysis was performed using SPSS statistical software VERSION 21. Data was analysed and expressed as frequencies and percentages. The sensitivity and specificity of Anti-CCP, RF FACTOR, RF IgM ELISA were calculated using Non-parametric tests (McNemar test) keeping the clinical diagnostic criteria as the gold standard. The p-value of <0.05 was considered statistically significant.
Results
A total of 60 patients clinically diagnosed with RA (including both males and females) were enrolled in the study after fulfilling ACR/EULAR criteria. Majority of the study participants were female constituting 85%, Male/female ratio-1:5.6. Mean age of presentation in RA group was 30±5 years age [Table/Fig-1].
Age wise (years) distribution of the patients.
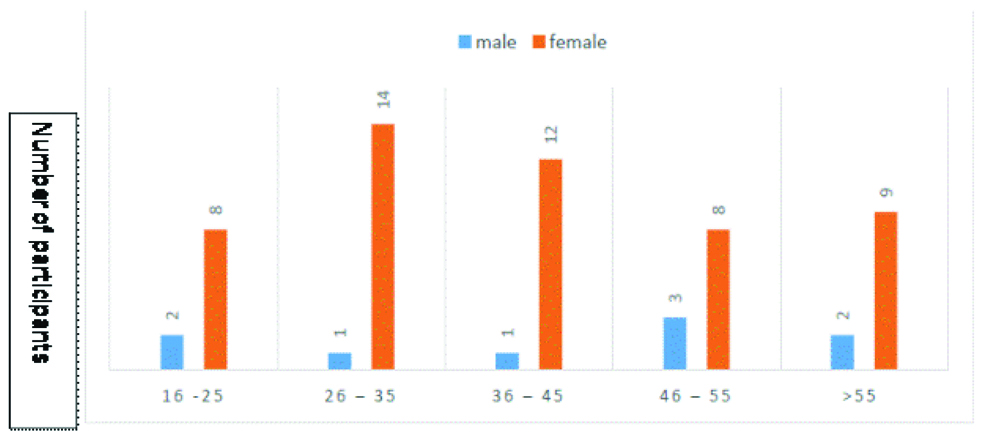
RF latex agglutination was positive in 39 (65%) out of 60 cases in the study group and was negative in 21(35%) out of 60 cases. RF detection by IgM ELISA was positive in 41 (68.3%) and negative in 19 (31.7%). Anti-CCP ELISA was positive in 42 (70%) and negative in 18 (30%). The results are tabulated in [Table/Fig-2].
Results of RF Latex, ELISA and Anti-CCP Tests (n=60).
| Tests | Positive | Negative |
|---|
| RF Latex | 39 | 21 |
| RF IgM ELISA | 41 | 19 |
| Anti-CCP | 42 | 18 |
Out of the 60 samples tested 30 (50%) were found to be positive by all three test methods. Nine samples (15%) were positive by both RF Latex and RF IgM ELISA. Only 2 (3.3%) samples were positive by both RF IgM ELISA and Anti-CCP. Anti-CCP alone was positive for 10 (16.7) samples in which the other 2 tests were negative. All three tests were negative in 9 (15%) samples as shown in [Table/Fig-3]. In the present study, 10 out of 21 (47.6%) RF seronegative patients were positive for Anti-CCP. Both RF ELISA and Anti-CCP had a higher sensitivity of 47.5% and 50% when compared to RF Latex which had a sensitivity of 42.9% [Table/Fig-4]. Also, the specificity of Anti-CCP and RF ELISA were higher, that is 82.4% and 80.4% when compared to RF latex which had a specificity of 76.5%.
Comparison of RF latex, RF IgM and Anti-CCP.
| Test | No. of samples | Percentage positive |
|---|
| RF Latex, RF IgM ELISA and Anti-CCP positive (All 3 tests positive) | 30 | 50% |
| RF latex and RF IgM ELISA positive (only 2 tests positive) | 9 | 15% |
| RF IgM ELISA and Anti-CCP positive (only 2 tests positive) | 2 | 3.3% |
| Anti-CCP only positive | 10 | 16.7% |
| RF Latex, RF IgM ELISA and Anti-CCP negative All 3 tests Negative | 9 | 15% |
| Total | 60 | 100% |
Sensitivity and Specificity of RF latex, Anti-CCP and, RF IgM ELISA.
| RA factor Latex agglutination-cross tabulation |
|---|
| Disease | RA factor | Total |
|---|
| Negative | Positive |
|---|
| Non RA | 9 | 0 | 9 |
| RA | 12 | 39 | 51 |
| Total | 21 | 39 | 60 |
| Sensitivity of RA factor Latex | 42.9% |
| Specificity of RA factor Latex | 76.5% |
| Anti-CCP-cross tabulation |
| Disease | Anti-CCP antibodies | Total |
| Negative (0) | Positive (1) |
| Non RA | 9 | 0 | 9 |
| RA | 9 | 42 | 51 |
| Total | 18 | 42 | 60 |
| Sensitivity of Anti-CCP ELISA | 50% |
| Specificity of Anti-CCP ELISA | 82.4% |
| RA factor IgM ELISA cross tabulation |
| Disease | Anti-CCP antibodies | Total |
| Negative (0) | Positive (1) |
| Non RA | 9 | 0 | |
| RA | 10 | 41 | |
| Total | 19 | 41 | |
| Sensitivity of RF IgM ELISA | 47.5% |
| Specificity of RF IgM ELISA | 80.4% |
Discussion
RA is a systemic autoimmune disease with few specific auto antibodies. ACR criteria include RF as a serological test for RA despite its low specificity [7]. Early control of inflammation in RA reduces the joint injury, thereby preventing irreversible damage. This mandates the need for early diagnosis and confirmation of RA and to avoid unnecessary treatment with anti-rheumatic drugs [5].
The present study was carried out to determine the sensitivity and specificity of three different methods (RF IgM Latex Agglutination test, RF IgM ELISA and Anti-CCP) in patients suspected to have RA. Among the 60 study participants, 51 were diagnosed as Rheumatoid arthritis and 9 as non-Rheumatoid arthritis (Early synovitis, Osteo Arthritis). This is similar to previous studies done by Das A et al., [16]. In this study there is a female predominance (85%) which was similar to the results of previous studies by Raza K et al., [6].
In our study, RF Latex had a 42.9% sensitivity which was similar to the study findings of Samanci N et al., 44.8% sensitivity [17]. However, a study by Machold KP et al., reported a higher sensitivity of 55% for RF test [18]. In contrast to the present study, a study by Dubucquoi S et al., showed a much higher sensitivity of 94% RF [19]. The specificity of RF by latex method in our study was 76.5%, which is higher than the findings of Vallbracht I et al., who report 86.4% [20]. This difference may be due to low sample size, short period of study and type of kits used for testing.
The sensitivity of RF IgM ELISA was 47.5% which correlated with the findings of Lakos G et al., who reported a sensitivity of 44.5% [21]. Kokkonen H et al., reported a slightly lower sensitivity of 32.8% [22]. The specificity of RF IgM ELISA was (80.4%) in our study, which correlated well with 86.4% reported by Samanci N et al., [17]. A higher specificity of 97.7% was observed by Lakos G et al., [21].
In the present study, Anti-CCP was positive in 70% of the patients and this was higher than those reported by Machold KP et al., and Bizzaro N et al., [18,23]. In this study, the sensitivity of Anti-CCP was 50% which did not correlate with that of Dubucquoi S et al., 77% sensitivity [19]. However, the specificity for Anti-CCP was 82.4%, which was much lower than the findings of Vallbracht I et al., 97.1% [20].
In the present study, 10 out of 21 (47.6%) RF seronegative patients were positive for Anti-CCP, this does not correlate with the findings of Vallbracht I et al., who report an overall positivity of 34.5%. [20], 40% of seronegative patients were Anti-CCP-positive which substantiates the additional diagnostic potential of Anti-CCP in seronegative patients.
In the present study, RF latex agglutination, RF IgM ELISA and Anti-CCP were positive in 50% of 60 patients. The results are comparable with the study by Vallbracht I et al., [20] who showed 59.3% positivity and are higher than the 39% positives reported by Schellekens GA et al., [10].
Thus, the present study suggests that an accurate early diagnosis of RA can be possible by a combination of RF ELISA and Anti-CCP rather than relying on a single serological test. RF IgM ELISA has the advantages of quantifying the amount of antibodies present apart from its detection which can act as the prognostic indicator in the subsequent treatments.
Limitation
This was a cross-sectional study, with small sample size and no follow-up of the patients were done. A larger sample size and a longitudinal study design would have been more beneficial in assessment of the RF IgM and Anti-CCP tests in diagnosis of RA.
Conclusion
The present study suggests that the combination of RF ELISA or Latex with Anti-CCP tests is a better diagnostic test for detection of RA.