Chronic lymphocytic Thyroiditis is a common autoimmune disease exhibiting marked lymphoid infiltrate destroying the thyroid follicles, which are eventually replaced by fibrosis. The disease is characterised clinically by an active phase which is transient exhibiting clinical manifestations of hyperthyroidism followed by evolution and destructive phase that manifest with subclinical or overt hypothyroidism [1].
Though worldwide the commonest cause of hypothyroidism is iodine deficiency, Hashimoto’s (lymphocytic) thyroiditis remains the commonest cause of spontaneous hypothyroidism in areas of adequate iodine intake. The annual incidence of lymphocytic thyroiditis worldwide is estimated to be 0.3-1.5 cases per 1000 persons exhibiting a female predilection, with peak incidence occurring in 30-50 years of age [2,3,4].
Fine needle aspiration cytology (FNAC) is a highly sensitive tool in diagnosing Hashimoto’s (lymphocytic) thyroiditis, with a diagnostic accuracy rate of 92% [5]. The diagnosis of the disease on fine needle aspiration cytology (FNAC) smears is made by finding the oxyphilic transformation of follicular epithelial cells (Hurthle cells), infiltration of follicles by lymphocytes and plasma cells, presence of moderate number of lymphoid cells in background with scanty or absent colloid in the background [6].
The present study was carried with an aim to study the clinical presentation, and cytomorphic spectrum of lymphocytic thyroiditis and correlate the cytological grades with the levels of TSH, FT3, FT4 and Anti-TPO antibodies. The concept was designed to enhance understanding of pathogenesis of the disease.
Materials and Methods
This study was carried out in the Department of Pathology at SGRRIM & HS, Dehradun, Uttarakhand, India. Approval of the study was taken from the institutional ethical committee (Registration No. ECR/710/Inst/Uk/2015) before commencing the study (Reference No.SGRR/IEC/52/16).
This was a prospective time bound analytical observational study and all the patients who presented to the hospital and fitted in the inclusion criteria were included. All patients aged between 12 to 80 years presenting to ENT/surgery OPD with painful or painless neck swelling and/or clinical features of constipation, lethargy or cold intolerance suggesting hypothyroidism or poor attention span, hyperactivity, restlessness, heat intolerance, loose stools suggesting hyperthyroidism were referred to pathology department for cytological diagnosis. Thus, all the cases of lymphocytic thyroiditis diagnosed on FNAC from June 2016 to May 2018 for which biochemical parameters were available were included in the study. Patients having history of thyroid related drug intake or any thyroid related surgery were excluded from the study. The relevant clinical details of the patient were included and recorded on predesigned proformas.
Fine-needle aspiration cytology (FNAC) of thyroid was done by using 23G needle attached to 10 mL syringe by the non aspiration technique [6]. Both air dried and wet fixed smears were prepared. The slides were stained with Giemsa (MGG) stain and Papanicolaou stain respectively. Fifty eight cases were cytologically diagnosed as lymphocytic (Hashimoto’s) thyroiditis on cytology. The smears were seen by two cytopathologists and grading was done independently based on the presence of impingement or infilteration by lymphocytes and plasma cell into the thyroid follicles, Hurthle cell change, Multinucleateb giant cell, epithelioid cell clusters and/or formation of lymphoid follicles. Grading of thyroiditis was done on the smears based on a set of predefined criteria as described by Bhatia A et al., into Mild (Grade-1) with few lymphocytes infilterating the follicles, Moderate (Grade-2) with moderate lymphocytic infilteration along with Hurthle cell change and Severe (Grade-3) with florid lymphocytic infilteration and formation of germinal centers [1]. The FNAC findings were correlated with histopathology reports, where surgical specimens were available specially to confirm the diagnosis and to exclude follicular or papillary neoplasm.
Statistical Analysis
The cytological features, TSH, Free T3, Free T4 and anti thyroid peroxidase (Anti-TPO) antibodies were statistically analysed for correlation by using One way ANOVA and Boferroni post Hoc test using SPSS version 17 for Windows 15.0 program and p-value of <0.05 was considered significant.
Results
Fifty eight cases of cytologically diagnosed cases were subjected to detailed biochemistry for confirmation. FT3, FT4, TSH and Anti-TPO values were available only in 31 cases for correlation and was done by a microplate enzyme immunoassay as it is highly specific, highly sensitive and less costly. The remaining cases did not turn for follow-up and biochemistry evaluation and were thus excluded from the study. The lack of complete data for all variables in all patients has reduced the sample size in the analysis of the various parameters thus statistical correlation was done for cytologically diagnosed and quantitatively graded 31 cases with the FT3, FT4, TSH and Anti-TPO. Of thirty-one cases of lymphocytic (Hashimoto’s) thyroiditis included in the study, majority of the patients (30 cases, 96.8%) were females. The most common age group was ≤40 years (24 cases, 77.4%), but the disease was also reported in older patients beyond 60 years [Table/Fig-1].
Distribution of study patients, according to age and sex.
| Sex | Total |
|---|
| Female | Male |
|---|
| No. | % | No. | % |
|---|
| Age group | ≤40 years | 23 | 95.8 | 1 | 4.2 | 24 |
| 41-60 years | 6 | 100 | 0 | 0.0 | 6 |
| >60 years | 1 | 100 | 0 | 0.0 | 1 |
| Total | 30 | 96.8 | 1 | 3.2 | 31 |
The cytomorphologic features of lymphocytic thyroiditis were examined and it was observed that 100% cases showed lymphocytes in the background and their infiltration into the thyroid follicular cells. Other characteristic features of lymphocytic thyroiditis like anisokaryosis and hurthle cell change were observed in 83.87% of cases. However, granuloma formation was the least significant finding and was observed in 5 cases (16.12%) [Table/Fig-2].
Cytomorphological features of lymphocytic thyroiditis (n=31).
| Cytological features | Number of cases | Percentage |
|---|
| Thyroid epithelial cells | 31 | 100% |
| Follicular lymphocytic infiltration | 31 | 100% |
| Background lymphocytes | 31 | 100% |
| Anisokaryosis | 26 | 83.87% |
| Hurthle cells | 26 | 83.87% |
| Giant cells | 12 | 38.70% |
| Epitheliod like cells | 12 | 38.70% |
| Granuloma | 5 | 16.12% |
| Germinal center formation | 7 | 22.58% |
| Colloid | 25 | 80.64 |
| Histiocytes engulfing colloid | 9 | 29.03% |
| Plasma cells | 9 | 29.03% |
The percentage distribution of patients according to age and cytological grades is shown in [Table/Fig-3]. Grade I thyroiditis was more commonly reported in 41 to 60 years of age group whereas as grade II and III were seen maximally in patients of 40 years or younger age group correlating with the peak age range which is 30-50 years for Hashimoto’s thyroidits to occur in females.
Distribution of patients, according to age and cytological grade of Lymphocytic Thyroiditis.
| Lymphocytic Thyroiditis | Total |
|---|
| Grade I | Grade II | Grade III |
|---|
| No. of cases | % | No. of cases | % | No. of cases | % |
|---|
| Age group | ≤40 years | 1 | 4.2 | 18 | 75 | 5 | 20.8 | 24 |
| 41-60 years | 2 | 33.3 | 2 | 33.3 | 2 | 33.3 | 6 |
| >60 years | 0 | 0.0 | 1 | 100 | 0 | 0.0 | 1 |
| Total | 3 | 9.7 | 21 | 67.7 | 7 | 22.6 | 31 |
FT3, FT4 and TSH was determined and post hoc test was applied on mean values for FT3, FT4 and TSH in different cytological grades of Lymphocytic thyroiditis, the mean difference among the different pairs of three groups was not found to be statistically significant (>0.05) [Table/Fig-4].
Post Hoc test for comparison of mean values of Thyroid Profile.
| Grade I and Grade II | Grade I and Grade III | Grade II and Grade III |
|---|
| FT3 | Mean difference | -3.71 | 0.51 | 4.21 |
| Standard error | 4.99 | 5.58 | 3.53 |
| p-value | 1.00 | 1.00 | 0.72 |
| FT4 | Mean difference | -5.52 | 5.54 | 11.06 |
| Standard error | 13.29 | 14.86 | 9.39 |
| p-value | 1.00 | 1.00 | 0.74 |
| TSH | Mean difference | -13.97 | -9.78 | 4.19 |
| Standard error | 22.43 | 25.08 | 15.86 |
| p-value | 1.00 | 1.00 | 1.00 |
On comparing cytological grade with Anti-TPO titer in serum and applying Post Hoc test, p-value was calculated. The p-value was statistically insignificant in the pair of grade I and II. But in pairs of grade I & III and grade II & III p-values was statistically significant. This shows that Anti-TPO is strongly associated with lymphocytic thyroiditis but its values are not correlated with the severity of the disease [Table/Fig-5].
Post Hoc test for comparison of mean difference of Anti TPO.
| Mean difference | Standard error | p-value |
|---|
| Grade I and Grade II | -1.29 | 708.17 | 1.00 |
| Grade I and Grade III | -2002.34 | 791.76 | 0.05 |
| Grade II and Grade III | -2001.06 | 500.75 | 0.001 |
Thus it was observed in our study that lymphocytic thyoiditis is commonly found in younger females. Though the positivity for antithyroid antibodies is strongly associated with lymphocytic thyroiditis, but cytomorphological grades, showed no correlation with the serum Anti-TPO titer or thyroid function test i.e. FT3, FT4 and TSH level.
Discussion
Chronic lymphocytic thyroiditis was described by Hakaru Hashimoto in 1912. Hashimoto’s thyroiditis is also considered a synonym of chronic lymphocytic thyroiditis or autoimmune thyroiditis including atrophic and non-goitrous thyroiditis [7]. It is considered to be an autoimmune disease characterised by activation of CD4+T cells which initiate recruitment of auto-reactive B cells which elaborates variety of thyroid autoantibodies. The principal biochemical characteristic of the disease is presence of autoantibodies Thyroid peroxidase (TPO) and Thyroglobulin (Tg) in the patient’s sera, against two major thyroid antigens [8,9].
Lymphocytic thyroiditis can affect any age group, but in the present study, the commonest age group was less than 40 years which is in concordance with studies by other authors, who have opined that the disease most commonly affects patients in 21-30 years of age group. The occurrence of disease in young patients is due to iodine deficiency in non coastal areas which is still prevalent despite national iodine deficiency diseases control program. In elderly, the disease may be seen in iodine sufficient areas. Many authors have linked increased incidence of HT particularly in coastal areas due to excess intake of iodine [1,10,11].
A female predominance was noted in the present study which is similar to observations by other authors who have noticed a female predilection for the disease. Besides the occurrence of disease in females has a early onset while it usually presents at a late age group in males [1,10,11].
The most common clinical presentation in present study was diffuse thyroid swelling which was seen in twenty seven patients (87.09%) while only four patients (12.9%) presented with nodular disease. This is comparable with the observations made by Anila et al., [12]. But this is significantly higher when compared to a study done by Bhatia A et al., where, only 2.63% of patients presented with nodular disease [1]. The authors have opined that nodular disease is usually representing the early stages of Hashimoto’s (lymphocytic) thyroiditis. But the patient usually reports to the physician in the advance stage of the disease, where the clinical and hormonal changes have already been established and patient clinically presents with diffuse thyroid swelling [12]. This is supported by our observation that in the present study a normal TSH values was observed in 5 cases (16.12%) while Anti-TPO value was raised in 26 cases (83.87%) and nodular disease was observed in four cases (12.9%).
Most of the patients presented with features of hypothyroidism i.e. weight gain (38.7%), hypothermia (41.9%) and fatigue/lethargy (38.7%). On hormonal assay, seventeen (54.80%) patients showed hypothyroidism, suggesting an advanced stage of the disease at the time of diagnosis and represented destructive phase of the disease. There were nine cases of hyperthyroidism (29.0%) in the study indicating Hashitoxicosis whichis a transient hyperthyroid phase. It is due to acute aggravation of thyroid autoimmunity induced destruction of thyroid follicles. Further, five cases were euthyroid (16.20%) with normal T3 and T4 levels indicating disease in phase of evolution. This is similar to and corroborate with studies by Kumar N et al., and Singh N et al., in which majority of the patients were hypothyroid [10,11]. On cytology L: E ratio (lymphoid: epithelial ratio) in Hashimoto’s thyroiditis range from 2:1 to 10:1 [11]. In the present study, all the 31 cases (100%) showed lymphocytes in the background with infiltration into the thyroid follicular cells. Other features like anisokaryosis and hurthle cell change were observed in twenty six cases (83.87%), giant cells and epitheliod like cells were seen in twelve cases (38.07%), plasma cells were observed in nine cases (29.03%) and germinal centre formation was observed in seven cases (22.58%).
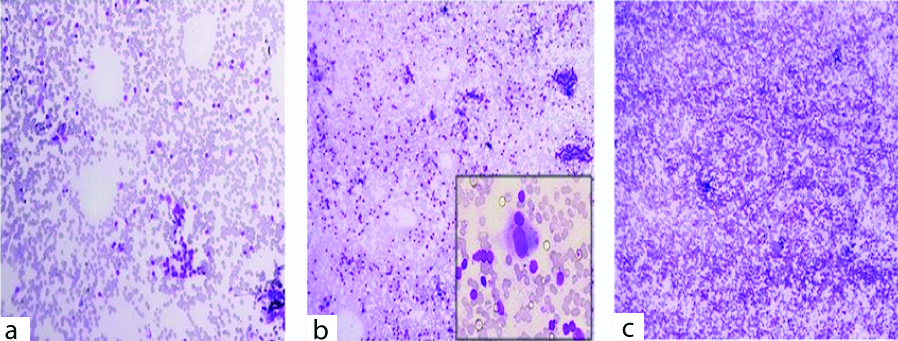
The cytological grading was done and it was observed that, three (9.7%) patients were of grade-I thyroiditis on cytology. The smears of these patients showed the presence of increased number of lymphocytes in the background or the lymphoid cells were noted to infiltrate thyroid follicular cells [Table/Fig-6a]. Smears of twenty one patients (67.7%) with grade-II thyroiditis showed the presence of Hurthle cells, epithelioid cells, giant cells, anisonucleosis and increased number of lymphocytes [Table/Fig-6b]. Seven cases (22.6%) of grade-III thyroiditis were characterised by the presence of florid lymphocytic infiltration with germinal center formation and the presence of scanty follicular cells [Table/Fig-6c]. The disease presents with two basic patterns that correspond to different phases of the disease and are readily recognised on cytology (I) Classic HT: seen in older age group who are more often hypothyroid and show increased lymphocytes in the background and infiltrating the follicular cell clusters. (II) Florid lymphocytic pattern: seen in younger age group and show predominance of lymphocytes in various stages of maturation. Epithelial cells may be inconspicuous [13]. It is now widely accepted that lymphocytic thyroiditis and HT represent different manifestations of autoimmune thyroiditis, however many authors use the term synonymously. In our study, majority of patients presented with grade-II disease which is supported by the studies done by Bhatia A et al., and Jayaram G et al., [1,14]. However, in the study by Anila K et al., majority of the patients presented with grade-I disease [12].
a) Grade I thyroiditis, increased number of lymphocytes in the background, (MGGX100); b) Grade II thyroiditis, moderate lymphocytic infiltration (MGGX100) and inset showing Hurthle cells (MGGX400); c) Grade III thyroiditis, florid lymphocytic infiltration (MGG X100).
Hurthle cell is a diagnostic feature of lymphocytic (Hashimoto’s) thyroiditis highlighting moderate to marked anisonucleosis and is described in wide range (48-98%) of lymphocytic thyroiditis by various authors [4]. In the present study we encountered Hurthle cell change in twenty six cases (83.87%), which were similar to the observation made by Singh N et al., [11].
This study showed elevated anti-thyroid peroxidase antibody (Anti-TPO) values in twenty cases (83.87%), all of these patients had characteristic cytological features of lymphocytic thyroiditis on smears. Detection of autoantibodies against Tg and TPO antigens are clinically most important for diagnosis and are found to be elevated in up to 95% of the patients [15]. It is however controversial to establish a diagnosis of the disease based solely on raised anti TPO. Anti-TPO positivity correlates strongly with cytological diagnosis of lymphocytic thyroiditis (p-value=0.001 to 0.05) but its values are not correlated with the severity of disease. In a study by Guarda LA and Baskin HJ, the antibody positive cases were found to be morphologically indistinguishable from seronegative cases [16].
Though the present data indicate no significant statistical correlation between the cytological grades and FT3, FT4, TSH, and Anti-TPO values, we feel that this study is limited by the sample size and a larger sample size is required to provide a more reliable data. On applying post hoc test on mean values for FT3, FT4 & TSH in different cytological grades of Lymphocytic thyroiditis, the mean difference among the different pairs of three groups was not found to be statistically significant (>0.05).
Singh N et al., included a larger sample size of 150 cases. It was observed that the grading of lymphocytic thyroiditis showed no correlation with the clinical severity of Hashimoto’s thyroiditis, while a high lymphoid: epithelial (L: E) ratio was strongly correlated with thyroid peroxidase positivity (p=0.004) [11].
However in the study by Bhatia A and Jayaram G et al., wherein the cytological grades were correlated with clinical, biochemical, ultrasonographic, and radionuclide parameters no significant statistical correlation was observed [1,14].
Anila K et al., also studied cytological grades of lymphocytic thyroiditis, TSH, Anti-TPO and thyroglobulin antibody values but failed to establish any significant correlation between the cytological grades and these biochemical parameters [12]. [Table/Fig-7] summarises the observations and comparisons of Lymphocytic thyroiditis with other studies.
Comparison between Present and the Previous studies.
| No. of patients | Age (year) | Sex | Cytological diagnosis | Clinical presentation (%) | Anti-TPO (%) | Cytological grading (%) |
|---|
| Bhatia A et al., [1] | 76 | 6-60 | 70 F6 M | 75 cases-Lymphocytic thyroiditis | Diffuse-89.47Nodular-2.63 | 65.7 | I-38.67II-44III-17.33 |
| Anila K et al., [12] | 60 | 5-74 | 55 F5 M | 60 cases-Lymphocytic thyroiditis | Diffuse- 77Nodular- 23 | 95 | I-45 II-36.67III-18.33 |
| Jayaram G et al., [14] | 40 | 40-50 | 40 F | 37 cases-Lymphocytic thyroiditis | _ | 57.5 | I-13.51II-62.16III-24.32 |
| Present study | 31 | 13-66 | 30 F1 M | 31 cases-Lymphocytic thyroiditis | Diffuse-87.09Nodular-12.90 | 83.8 | I-9.7II-67.7III-22.6 |
It is a well known fact and can be observed from studies by other authors that localized intrathyroidal immune destruction begins much earlier than serologic evidence of disease. Thus, antibody titer may be variable in the course of disease but cytomorphologic features persist during course of lymphocytic thyroiditis.
Limitation
The procedure of making a diagnosis of lymphocytic thyroiditis on FNAC is superior though has many limitations. There may be an overlap of cytological picture with Hashimoto’s thyroiditis, and a mixed inflammatory infilterate in background might be an obvious finding instead of pure lymphoid inflammation.
Encountering numerous Hurthle cells on cytology may result in an erroneous diagnosis of Hurtle cell neoplasm [17]. Careful sampling and searching for lymphocytes in Hurthle cell cluster in addition to other cytological findings will lead to correct diagnosis. In event of Florid lymphocytic thyroiditis, the cytological findings may be simulate lymphoma which needs to be excluded by careful sampling and interpretation. Occurrence of thyroid lymphomas in the setting of lymphocytic thyroiditis is a well known finding. Autoimmune thyroiditis may induce monoclonal proliferation of lymphocytes thus leading to the development of mucosa associated lymphoid tissue lymphoma, which can lead to an aggressive lymphoma [18]. Autoimmune thyroiditis may be associated with other autoimmune disorders as is well documented in previous studies [19,20]. Other modalities of investigations like thyroid isotope scanning, radio-iodine uptake test, USG, CT, fluorescence scanning, molecular markers when employed enhances diagnostic reproducibility but was not possible in our setting due to cost factor.
Conclusion
To conclude we are of the opinion that the lymphocytic thyroiditis should be diagnosed by a multidisciplinary approach. Many patients with lymphocytic thyroiditis may have neither symptoms nor physical signs of the disease. Thus, Clinical features and serum findings when used alone to make a diagnosis may result in missed diagnosis. Clinical, biochemical, cytological and radiological parameters should be taken into consideration together to reach a final diagnosis. However, in spite of the availability of different diagnostic modalities, demonstration of lymphocytic infiltration by fine needle aspiration cytology still remains the gold standard.
Thus biochemical parameters are an adjunct to cytomorphological diagnosis and should be used to enhance the diagnostic accuracy and reproducibility. It was observed that as the grade of HT increases, the probability of hypothyroidism increases. This suggests that the chronicity of the disease increases the chances of hypothyroidism increases. Though there is strong association of antithyroid antibodies, especially Anti-TPO with Lymphocytic thyroiditis, the present study and previous similar studies have failed to establish any significant correlation between the cytological grades and these biochemical parameters. Cases with raised TPO and normal TSH should have a close follow-up as to avoid deleterious effects of the disease because of known association of the disease with malignancy and to ensure a confident diagnosis of thyroiditis. Besides, the coexistence of goiter and hypothyroidism necessitates further evaluation of disease despite intake of fortified salt.
With this background, Fine Needle Aspiration Cytology (FNAC) findings and assay of serum Anti-Thyroid Peroxidase (anti-TPO) antibody have evolved as major components in the investigations of thyroid nodules and diffuse thyroid enlargement.
Future recomendation
A longitudinal study with larger sample size may enhance diagnostic reliability besides providing some additional findings, especially regarding stages of autoimmune thyroiditis and its relation with Anti-TPO antibody level.