Localisation of the prostate using gold markers has emerged as standards of practice in its positional identifications and target tracking in three dimensions. Daily treatment verification with Cone Beam Computed Tomography (CBCT) ensures the conformity and reproduction of PTV and could potentially reduce the PTV by opting for tighter margins. This could translate into a reduction of acute and late radiation toxicity with resultant dose reductions to Organ At Risk (OAR) without affecting the biochemical control.
The dosimetric superiority of PTV margin reduction using Tumour Control Probability/Normal Tumour Complication Probability (TCP/NTCP) model with a possible reduction in late radiation toxicity has been demonstrated in a number of studies [2,3]. However, translating the theoretical and dosimetric advantage of this plausible PTV reduction in a clinical setting without compromising clinical outcome remains debatable. Chaurasia A et al., and Crehange G et al., have attempted reduction of PTV margin in clinical scenario using 3D ultrasound system (SonArray TM) [4,5]. Limited data exist with studies evaluating PTV reduction using fiducial marker based IGRT formed the basis to conduct this study.
In the present institution, gold marker based IG-IMRT for prostate radiotherapy is being practised since 2013. As an institutional protocol, a uniform PTV margin of 7 mm was taken except posteriorly where a customised 6 mm margin was tailored to meet the target volume requirements till 2014. Based on our own dosimetric data and daily CBCT image verifications of set-up errors and prostate motion variability, we changed our institutional protocol to 5 mm uniform PTV margin and daily image guidance was mandatorily practised and recorded using online verification protocol. The present study aimed to retrospectively evaluate and report the effect of PTV reduction on the acute as well as late radiation toxicity. Also, authors intend to compare the clinical outcome in terms of biochemical control in this subgroup of patients.
Materials and Methods
The present study is a single institution retrospective evaluation conducted in Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India, from August 2013 to June 2017 with due approval from the Institutional Ethics Committee (IEC: 2017/admin-12-7, dated 21-06-2017). Patients with histopathologically proven prostate cancer, T1c-T3b with N0M0, age 18-80 years, Karnofsky performance status of ≥70 with no prior history of chemotherapy or radiotherapy were included in the study [6]. Detailed history, clinical examination, complete blood count, serum biochemistry including serum Prostate Specific Antigen (PSA) levels were done. Contrast Enhanced Magnetic Resonance Imaging (CEMRI) of the pelvis was done as part of pretreatment work-up in all patients. Further, contrast enhanced computed tomography of thorax and abdomen and bone scan was performed to rule out metastatic disease.
They were staged according to the American Joint Committee on Cancer (AJCC) recommendations, 7th edition (2010) [7]. Risk groups were determined by the D’Amico AV et al., risk stratification [8].
All the patients received neoadjuvant, concurrent and adjuvant hormonal therapy with leuprorelin 11.5 mg monthly subcutaneously or bilateral orchidectomy along with tablet bicalutamide 50 mg once daily for first three months for complete androgen blockade. The hormonal therapy was given for 6 months in intermediate risk patients, whereas for high-risk patients it was continued for two years. In PTV_7 arm bilateral orchidectomy was done in 14/25 patients, rest received injection leuprolide. In PTV_5 arm bilateral orchidectomy was done in 16/25 patients.
Three fiducial gold markers (Cyber MarkTM Fiducial Marker Kit; CIVCO Medical Solutions, Kalona) measuring 1×5 mm were inserted by urologist under transrectal ultrasound guidance in three non-coplanar positions; that is, left superior lobe, left apex and right mid-gland.
Simulation
After a period of seven days of fiducial placement, contrast enhanced Computed Tomography (CT) simulation was performed thus allowing enough time for oedema to subside. As a part of rectal protocol, patients were given tablet Bisacodyl, two tablets a day before the simulation and were asked to come with empty rectum for treatment [9]. An institutional bladder protocol was followed, whereby the patients were advised to void the bladder followed by drinking 500 mL of water, 30 minutes prior to CT simulation [10]. This bladder protocol was followed during the entire course of radiotherapy and patients were also advised to empty bowel before each fraction of treatment. Prior to simulation scans, patients were immobilised in supine position with thermoplastic cast and knee rest. The planning CT scan was performed and 3 mm CT slice was obtained (from upper abdomen to mid-thigh) after injection of 100 mL of non-ionic contrast using multi-slice CT simulator (Somatom sensation; Siemens Medical Solution, Germany). Three iso-centrically placed laser centres with an accuracy of ±0.1 mm were used for localisation point to ensure accurate and reproducible positioning and this was tattooed on bony skin points. Standard DICOM protocol (3.0) was followed for image data transfer to Treatment Planning System (TPS, Monaco version 5.0).
Contouring
Target volume and OAR delineation were done as per International Commission on Radiation Units and Measurements (ICRU) Reports No. 50, 62 and 83 [11-13]. Contouring of primary target volumes was done according to the EORTC contouring guideline (Boehmer D et al.,) [14]. The nodal contouring was done in accordance with RTOG nodal contouring for prostate carcinoma [15]. The structures contoured included the prostate, seminal vesicle and the pelvic lymph node group. The HRCTV included prostate along with proximal seminal vesicle in intermediate risk patients. In high-risk patients, gross extracapsular volume was also encompassed in the HR CTV. Distal seminal vesicle and pelvic lymph nodes were included in the Low-risk CTV (LR CTV). In the PTV_7 arm, respective HR-PTV and LR-PTV were obtained by a uniform PTV expansion of 7 mm, except posteriorly, which received a 6 mm expansion. While in PTV_5 arm, uniform PTV expansion of 5 mm was used. Rectum (up to sigmoid colon), bladder, pelvic bone, bowel bag, femoral heads were contoured as OAR according to RTOG pelvic normal tissue contouring guidelines by Gay HA et al., [16]. Besides the three gold seeds were contoured in the CT slices where it was visible.
Planning
In high-risk group patients, RT was given to both primary disease (prostate+seminal vesicle) and bilateral pelvic lymph nodes. While in intermediate risk patients, only primary disease (prostate+seminal vesicle) was irradiated. The total dose delivered was 78 Gray in 39 fractions at 2 Gray per fraction over 8 weeks in 2 phases. In 1st phase, 46 Gray was delivered in 23 fractions at 2 Gray per fraction to both HR and LR PTV. A boost dose of 32 Gray in 16 fractions was delivered in 2nd phase to HR PTV.
Volumetric Modulated Arc Therapy (VMAT) planning was performed using TPS-CMS Monaco (Version 5.0). All treatment plans were evaluated and implemented only after meeting the stringent Quality Assurance (QA) parameters. Treatment was delivered on multi-energy (6-10-15 MV) ELEKTA Infinity (Crawley, UK) linear accelerator with dynamic multileaf collimation (40-pair MLC), with a leaf width of 1 cm at the isocentre. Prior to delivery of RT, the plans were rigorously evaluated which included Dose Volume Histogram (DVH) analyses and a slice by slice (axial and sagittal) analysis of the isodose lines to ensure that 90% isodose line encompassed the half width margin of the rectum and 50% isodose line incorporated the full width of the rectum on all axial slices.
For reproducibility, pretreatment KV-CBCT images of target volume were acquired by X-ray Volume Imaging (XVI) (version 4.5.1) using volume view and dual registration (click box registration facility) for all patients and for each fraction. The KV-CBCT data were recorded and evaluated.
Patients on RT were evaluated weekly for acute toxicities and were graded as per Common Terminology Criteria for Adverse Events (CTCAE) 4.03 [17] Late toxicities were graded as per Radiation Therapy Oncology Group (RTOG) late morbidity criteria [18]. Post RT, 1st follow-up was after four weeks. The patients were followed-up once a month for 1st year, every two months for next two years and every six months thereafter. Serum PSA value was assessed once in every three months.
Statistical Analysis
Statistical analysis was done with SPSS (statistical package for social sciences) software version 20.0. Mean and standard deviation were estimates of quantitative data. The prostate motion variability between two groups was compared using unpaired t-test. The acute and late toxicities (Grade <2 vs. Grade ≥2) were evaluated and compared between two arms by calculating the odds ratios (OR), with 95% confidence interval (95% CI) using the chi-square test/Fisher-exact test. Biochemical failure was defined as a rise by 2 ng/mL or more above the nadir PSA after EBRT and the date of failure was determined as per phoenix consensus guideline [19]. Biochemical Progression Free Survival (B-PFS) was calculated from date of biopsy untill biochemical failure. Survival rates were estimated using Kaplan-Meier method and log-rank test was used to compare survival outcomes. All reported p-values are two sided and value of ≤0.05 was considered significant.
Results
The study was conducted between November 2013 to July 2017 and 50 patients were evaluated (25 each in each arms). The baseline patient characteristics were similar in two arms and are depicted in [Table/Fig-1].
| Variable | Total n=50 | PTV_7 n=25 | PTV_5 n=25 |
|---|
| Median age (range) | 70 (55-90) | 69 (56-90) | 70 (55-81) |
| Median Karnofsky performance status (range) | 80 (70-90) | 80 (70-90) | 80 (70-90) |
| Gleason score |
| ≤6 | 13 | 6 | 7 |
| 7 | 24 | 12 | 12 |
| >7 | 13 | 7 | 6 |
| Tumor stage* |
| T1 | 1 | 0 | 1 |
| T2 | 20 | 9 | 11 |
| T3 | 29 | 16 | 13 |
| Pre RT PSA** levels |
| <10 | 5 | 3 | 2 |
| 10-20 | 9 | 4 | 5 |
| >20 | 36 | 18 | 18 |
| Risk stratification (D’Amico) |
| Intermediate | 4 | 2 | 2 |
| High | 46 | 23 | 23 |
| Total RT dose |
| 78 Gray/39# | 50 | 25 | 25 |
| 3 months post-RT PSA |
| <0.1 | 45 | 23 | 22 |
| 0.1-2 | 5 | 2 | 3 |
| CTV volumes (Median) |
| CTV primary volume (cc) | 68 | 68 | 66 |
| CTV total volume (cc) | 437 | 430 | 442 |
| BMI# median (range) | 23 (20-28.6) | 22.3 (20.6-26) | 23.6 (20-28.2) |
| RT duration median (range) | 57 (54-63) | 56 (54-59) | 57(56-63) |
*AJCC 7th staging, **PSA: Prostate specific antigen, #BMI: Body mass index, CTV: Clinical target volume
The treatment compliance was good in both arms with treatment interruption of >5 days observed in only two patients both in PTV_7 arm due to Grade 3 proctitis and diarrhoea. The RT planning constraints used and target parameters achieved during RT planning are illustrated in [Table/Fig-2] and were well balanced between two arms.
| Dose constraint | Prescribed | PTV_7 (n=25) Achieved (Mean±SD) | PTV_5 (n=25) Achieved (Mean±SD) | p-value |
|---|
| Bladder | V40≤50% | 47.1%±2.7 | 46.5%±2.4 | 0.41 |
| V65≤20% | 18%±0.8 | 17.5%±1.2 | 0.08 |
| Rectum | V40≤50% | 46.2%±2.9 | 44.8%±2.2 | 0.06 |
| V65≤25% | 22%±2.1 | 23%±1.9 | 0.08 |
| Head of femur | V50≤10% | 8.8%±0.3 | 8.6%±0.7 | 0.19 |
| Bowel bag | V45≤195 cc | 116 cc±42 | 126 cc±22 | 0.29 |
PTV: Planning target volume, V40: Volume receiving 40 Gray, V65: Volume receiving 65 Gray, V50: Volume receiving 50 Gray
Late Toxicity
The median follow-up was 37 months (11-76 months). The overall late rectal toxicity at three years was 72%, 18% and 10% for Grade 1, 2 and 3. Similarly, three year urinary toxicities were 68%, 24% and 8% for Grade 1, 2 and 3 respectively. The Grade ≥3 chronic GI toxicities In PTV_7 group were significantly higher compared to PTV_5 group (10% vs. 0%, p=0.05). The Grade ≥3 chronic GU toxicities were higher in PTV_7 group (6 % vs. 2%, p=0.60) which was found to be statistically insignificant. The acute toxicities have been enunciated in [Table/Fig-3]. The acute RT toxicities in terms of proctitis {48% vs. 16%, OR=4.84 (1.28-18.25), p=0.03} and gastrointestinal pain {44% vs. 12%, OR=5.76 (1.36-24.36), p=0.02} were significantly higher in PTV_7 arm compared to PTV_5 arm.
Maximal acute radiation reaction [Total n-50].
| Parameter | PTV_7 (n=25) | PTV_5 (n=25) | OR (95%CI) | p-value |
|---|
| Grade | n | Grade | n |
|---|
| Skin reactions | <2 | 22 | <2 | 24 | 3.27 (0.31-33.83) | p=0.60 |
| ≥2 | 3 | ≥2 | 1 |
| Diarrhoea | <2 | 18 | <2 | 21 | 2.04 (0.51-8.11) | p=0.49 |
| ≥2 | 7 | ≥2 | 4 |
| Fatigue | <2 | 19 | <2 | 20 | 1.25 (0.32-4.83) | p=1 |
| ≥2 | 6 | ≥2 | 5 |
| Gastrointestinal pain | <2 | 14 | <2 | 22 | 5.76 (1.36-24.36) | p=0.02 |
| ≥2 | 11 | ≥2 | 3 |
| Proctitis | <2 | 13 | <2 | 21 | 4.84 (1.28-18.25) | p=0.03 |
| ≥2 | 12 | ≥2 | 4 |
| Urinary frequency | <2 | 19 | <2 | 21 | 1.65 (0.40-6.78) | p=0.72 |
| ≥2 | 6 | ≥2 | 4 |
| Weight loss | <2 | 23 | <2 | 23 | 1 (0.12-7.71) | p=1 |
| ≥2 | 2 | ≥2 | 2 |
OR: Odds ratio, CI: Confidence interval
Biochemical Control
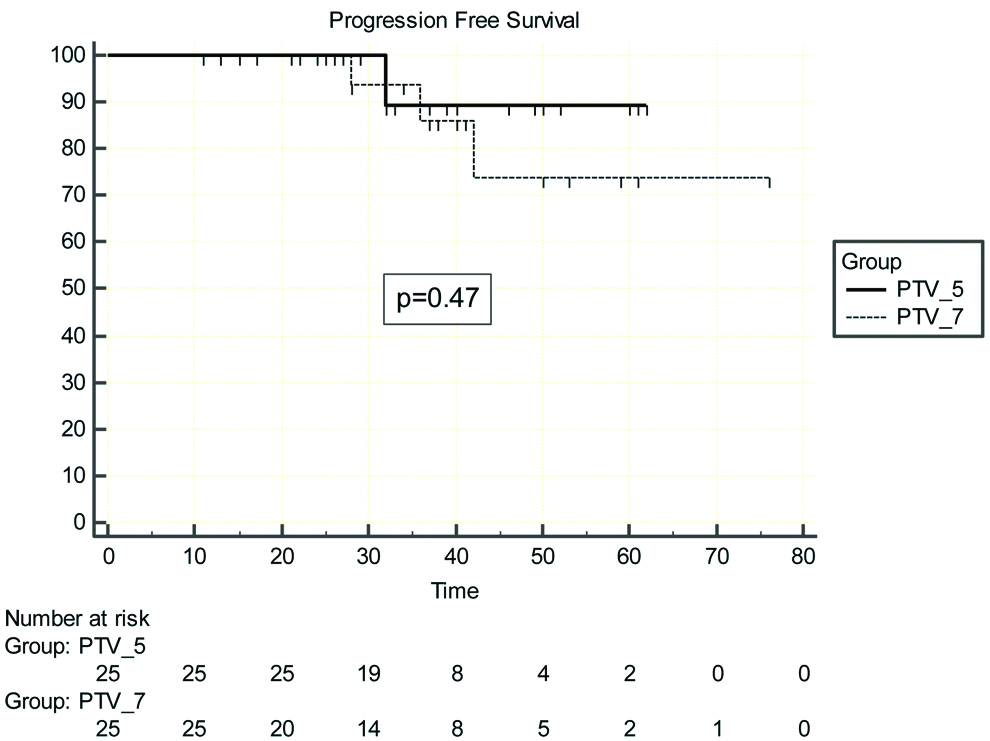
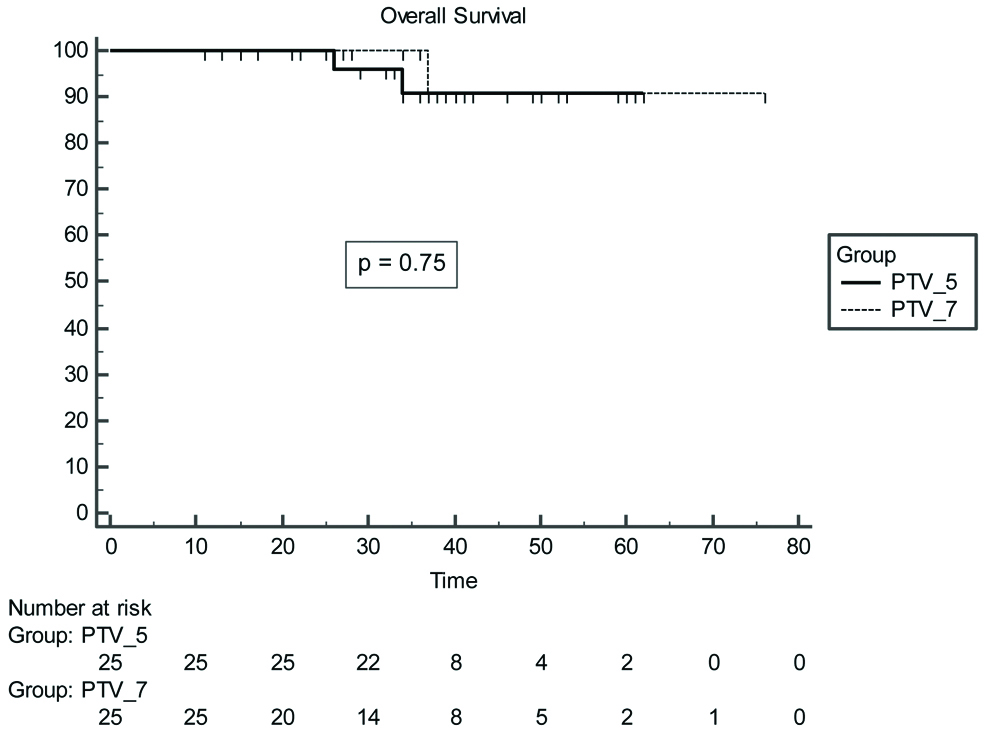
At a median follow-up of 37 months three year B-PFS rates were 89.5±7% (PTV_5) vs. 85.9±9% (PTV_7), p=0.47] [Table/Fig-4]. The median OS was 37 months whereas median B-PFS was 36 months. There were two deaths in PTV_5 arm, one patient initially developed biochemical progression followed by bone and liver metastasis, was treated with docetaxel based chemotherapy, but succumbed to disease after achieving partial response. The second patient died due to myocardial infarct (biochemically controlled till last visit). The three year OS rates were 100% for PTV_7 group vs. 90.8% for PTV_5 with p=0.75 [Table/Fig-5].
Shows progression free survival for PTV_7 vs. PTV_5 arm patients.
Overall survival for PTV_7 vs. PTV_5 arm patients.
Discussion
External beam radiotherapy in prostate carcinoma has undergone spectral changes in the last two decades from two-dimensional RT to highly conformal IG-IMRT. Dose escalation has been the emerging norm in prostate radiotherapy. Various large randomised controlled trials have proven that RT dose ascendance has been beneficial in terms of biochemical control with acceptable toxicities [20-23]. Addition of image guidance leads to better localisation, thus reducing uncertainties related to prostate motion variability of target volume [24-26]. This method could allow reduction of PTV margin. However, jury is out on whether such an attempt would take its toll on clinical outcomes and whether it can potentially reduce the volume of bladder and rectum receiving high dose of radiotherapy and subsequently reducing the acute and late radiation induced toxicity. Various published reports and undergoing studies address this issue.
Wen N et al., used three different PTV margins, 1st group with uniform PTV margin of 10 mm except 6 mm at the rectal interface (10/6 mm), 2nd group with 5 mm except posteriorly to 3 mm and in the 3rd group further reduced to 3 mm uniformly and evaluated predicted TCP and NTCP in the resultant accumulated doses in eight patients [2]. The average percent reductions in the predicted TCP and the mean increase in the predicted NTCP for Grades 2/3 rectal bleeding in the accumulated (actual) plans in comparison to the original plans were, 0.4%, 0.7% and 11.0%, and, 3.5%, 2.8% and 2.4%, respectively. Thus, it can be inferred from the findings that the present authors can yield higher quality treatments by individualising treatment plans through margin adaptation based on biological models. Maund IF et al., in a dosimetric study using TCP/NTCP model demonstrated the feasibility of PTV margin reduction to 3 mm uniformly without compromising tumour control [3]. They hypothesised that consequential normal tissue sparing results in reduced anticipated rectal toxicity [3].
In the present study, gold fiducial marker based IGRT has been utilised for daily verification of reproducibility of PTV and prostate motion tracking using Cone Beam Computed Tomography (CBCT). Cohort studies comparing ultrasound guidance and fiducial marker based IGRT, have revealed higher accuracy for prostate localisation with marker. Scarbrough TJ et al., have reported their findings of ultrasound based variability, which revealed larger systematic/random error compared to fiducial based data in all three-dimensions [27]. Thus, suggesting that larger PTV margin (9 mm) is required in ultrasound based IGRT as compared to 3 mm for fiducial marker based IGRT. A similar study by Johnston H et al., reinforced the advantage of fiducial markers over ultrasound based IGRT in terms of accuracy and reproducibility in 3D for prostate localisation [28]. In view of better precision for prostate localisation that is achieved with fiducial based IGRT, authors used it in the present patients.
The present authors used a uniform PTV margin of 5 mm in the PTV_5 arm. The optimal PTV margin in modern IGRT era has been evaluated in many studies and it still remains debatable. Skarsgard D et al., evaluated adequate margin with and without image guidance in 46 patients [29]. The study showed that without image guidance, the PTV margins required to cover 95% of target volume was 0.57 cm (left-right), 0.79 cm (anterior-posterior) and 0.77 cm (superior-inferior) respectively. However, with the addition of fiducial markers and daily imaging with Electronic Portal Imaging Device (EPID), these margins are reduced to 0.36 cm, 0.37 cm and 0.37 cm respectively. Schallenkemp JM et al., analysed relative prostate position to the pelvic bony anatomy for 20 patients with implanted gold fiducial markers with daily portal images [30]. The average prostate displacement improved from 2.5 to 1.4 mm, 3.7 to 1.6 mm and 1.9 to 1.1 mm in vertical, horizontal, and right-left axes respectively, with use of image guidance with all three differences being statistically significant (all p<0.001). Barney BM et al., analysed 1244 CBCT data in 36 patients and demonstrated that mean differences in the AP, SI, and LR dimensions were 3.4±2.6 mm, 3.1±2.7 mm, and 1.3±1.6 mm, respectively [31]. Difference of >5 mm which was observed in 28% initially was brought down to 5% after online corrections were implemented. Again another study revealed prostate variability with and without markers in millimeter to be 4.1±2.3 vs. 3.7±2.1 {Antero-Posterior (A-P); p=0.001}, 2.3±1.5 vs. 2.1±1.2 {Superior-Inferior (S-I); p=0.095} and 1.1±1.7 vs. 0.4±1.4 {Left-Right (L-R); p=0.003} [32]. Thus, illustrating that using daily marker based image guidance and subsequent online correction, a cut-off of 5 mm as the PTV margin can safely encompass 95% of the PTV volume.
Positional verification is a crucial part of delivery as the planning. Various methods have been developed for this ranging from, two dimensional portal verification to volumetric verification using CBCT. Pawlowski JM et al., and Ariyaratne H et al., in two dosimetric studies showed that PTV for prostate can be safely reduced to 5 mm using daily CBCT [33,34]. Ariyaratne H et al., evaluations further enunciated the dosimetric advantage of daily CBCT in place of weekly CBCT [34]. They showed that daily CBCT improves target coverage in 90% of patient and reduces rectal dose in 80% cases compared to weekly protocol. In a recent study Gupta M et al., advocated use of daily IGRT while using tighter PTV margins [35]. In this study, 5% increased risk of geographical miss was estimated with every 15% reduction in CBCT frequency. Based on the finding of these studies, the present authors preferred CBCT over EPID and daily CBCT over the weekly protocol for treatment verification.
Crehange G et al., evaluated the clinical impact of reducing the PTV margin from 10 mm to 5 mm in 165 patients with an escalated dose of 78 Gray using 3D-ultrasound based IGRT [5]. The study revealed that 3 years B-PFS was similar in the two arms (92.5% vs. 94.3%, p=0.84). At a median follow-up of 39 months, Grade 2 Genitourinary (GU) toxicity was 7.0% vs. 6.6%, p=1.00, Grade 2 gastrointestinal toxicity was 1.2% and 2.6% (p=0.38). Both GI and GU toxicities were in favour of small PTV margin, but it failed to reach the level of statistical significance. The authors inferred that PTV margins can safely be reduced without compromising on clinical outcome, but its influence on acute or late toxicity is yet to be established. The study by Chung HT et al., also demonstrated the advantage of adding image guidance by enabling PTV margin reduction and consequently reducing the acute RT toxicity [36]. A comparative analysis of acute and late toxicities in various studies using different PTV margins with that of ours has been enunciated in [Table/Fig-6] [5,36-38].
Comparative analysis of acute and late toxicity in different studies with different PTV margins.
| Study | PTV margin | Acute GI ≥grade 2 | Acute GU ≥grade 2 | Late GI ≥grade 3 | Late GU ≥grade 3 |
|---|
| Wortel (38) n=260 | 5-8 mm (IG-IMRT arm) | 29% | 38% | - | - |
| 10 mm 3D-CRT arm | 49% | 48% | - | - |
| Chung (36) n=25 | 3 mm circumferential (IGRT Arm) | 13% | 13% | - | - |
| 10 mm circumferential5 mm posteriorly(IMRT Arm) | 80% | 60% | - | - |
| Crehange (5) n=165 | 5 mm circumferential | - | - | 1.2% | 7% |
| 10 mm circumferential | - | - | 2.6% | 6.6% |
| Zelefsky (37) n=376 | 10 mm circumferential6 mm posteriorly IGRT | 1.1% | 18.4% | 1% | 10.4 |
| 10 mm circumferential6 mm posteriorlyNon-IGRT | 1.6% | 26.8% | 1.6% | 20% |
| Our study n=50 | 5 mm circumferential | 16% | 16% | 0% | 2% |
| 7 mm circumferential6 mm posteriorly | 48% | 24% | 10% | 6% |
PTV: Planning target volume, GI: Gastrointestinal, GU: Genitourinary, IG-IMRT: Image guided-intensity modulated radiotherapy, IGRT: Image guided radiotherapy, IMRT: Intensity modulated radiotherapy
There has been an ongoing debate about the use of IMRT or VMAT. In VMAT, one or multiple arcs are used which allows the simultaneous variation in gantry rotation speed, dose rate, and MLC leaf positions, unlike step-and-shoot IMRT where the MLC divide each radiation beam into a set of smaller segments of differing MLC shape and switching off beam between the segments thus taking longer on treatment time. Ariyaratne H et al., and Shelton J et al., have published their findings on the comparative advantage of VMAT over IMRT [34,39]. They emphasised the need for VMAT in place of seven fields or nine fields static IMRT in patients where smaller PTV margin is used to minimise treatment time thus ultimately reducing the intra-fraction prostate motion. However, dynamic MLC IMRT is less time-taking and delivers fewer monitor units in comparison to VMAT. Keeping these observations in mind, and taking into account the experience of our physics team with VMAT, VMAT technique was used in all the patients in our study cohort.
Limitation
The present study has the inherent limitation being retrospective in nature and not being a matched pair analysis. Relatively smaller follow-up, limits the generalisation of biochemical control to all patient sub-groups. In future studies, prospective evaluation of smaller PTV margins are warranted with a longer follow-up period to evaluate the late failure pattern. Further studies are needed to analyse the significance of margin reduction in hypofractionated RT in low to intermediate risk prostate carcinoma, where evidence is emerging rapidly.
Conclusion
Planning Target Volume (PTV) reduction can be safely used with dose escalated radiotherapy while using marker based IG-IMRT with daily verification. This results in a reduction of acute and possible decline in late GI toxicity without compromising on biochemical failure. However, a larger prospective study with a longer follow-up may shed further light on the long term influence of PTV reduction on late toxicity and outcome.
Declaration: The abstract was presented in poster session in 37th ESTRO conference 2018 at Barcelona, Spain with abstract number EP-1569.
*AJCC 7th staging, **PSA: Prostate specific antigen, #BMI: Body mass index, CTV: Clinical target volume
PTV: Planning target volume, V40: Volume receiving 40 Gray, V65: Volume receiving 65 Gray, V50: Volume receiving 50 Gray
PTV: Planning target volume, GI: Gastrointestinal, GU: Genitourinary, IG-IMRT: Image guided-intensity modulated radiotherapy, IGRT: Image guided radiotherapy, IMRT: Intensity modulated radiotherapy