Study to Find Out Predisposing Factors, Causality, Severity and Avoidability of Adverse Drug Reactions among Patients Treated under DOTS Centre of Northern India
Sachin Tutu1, Garima Adhaulia2, Suchi Jain3, Surya Kant4, Ajay Verma5, Amod Kumar Sachan6, Rakesh Kumar Dixit7
1 Resident, Department of Pharmacology and Therapeutics, King George’s Medical University, Lucknow, Uttar Pradesh, India.
2 Resident, Department of Pharmacology and Therapeutics, King George’s Medical University, Lucknow, Uttar Pradesh, India.
3 Resident, Department of Pharmacology and Therapeutics, King George’s Medical University, Lucknow, Uttar Pradesh, India.
4 Professor and Head, Department of Respiratory Medicine, King George’s Medical University, Lucknow, Uttar Pradesh, India.
5 Associate Professor, Department of Respiratory Medicine, King George’s Medical University, Lucknow, Uttar Pradesh, India.
6 Professor, Department of Pharmacology and Therapeutics, King George’s Medical University, Lucknow, Uttar Pradesh, India.
7 Professor, Department of Pharmacology and Therapeutics, King George’s Medical University, Lucknow, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Garima Adhaulia, Resident, Department of Pharmacology and Therapeutics, King George’s Medical University, Lucknow, Uttar Pradesh, India.
E-mail: drgarima.26@gmail.com
Introduction
Tuberculosis (TB) is among the top 10 leading causes of death globally. Association of Adverse Drug Reactions (ADRs) with Anti Tubercular Therapy (ATT) drugs has been encountered more commonly which causes significant morbidity and even mortality.
Aim
To find out the predisposing factors and to assess the causality, severity and avoidability of the ADRs in proven TB patients treated under Directly Observed Treatment Shortcourse (DOTS) regimen.
Materials and Methods
This study was conducted at the Department of Respiratory Medicine, King George’s Medical University, Lucknow. TB patients who were kept on DOTS regimen under Revised National Tuberculosis Control Programme (RNTCP), satisfying the inclusion and exclusion criteria with written informed consent, were recruited and monitored from May 2016 to April 2017. A total of 115 patients were enrolled for the study. TB diagnosis was confirmed by sputum smear. The treatment was initiated as per Category I-newly diagnosed patients of TB and Category II-previously treated patients pending drug sensitivity testing result (According to Treatment of TB: Guidelines, 4th edition, 2010, WHO, Geneva). The patients were followed up and monitored for suspected ADRs. For causality assessment Naranjo’s algorithm and WHO-UMC classification scales, for severity assessment Modified Hartwig and Siegel Scale according to severity levels, for avoidability assessment Halla’s scale were used. Categorical variables were presented in number and percentage (%). Chi-Square test/Fisher’s-exact test was used for the comparison of qualitative variables. The p-value of <0.05 was considered statistically significant.
Results
Out of 115 patients enrolled, 87 ADRs were observed in 67 cases. Incidence of ADRs were more in patients of TB with Diabetes Mellitus (DM) (79.16%) and with HIV (70.59%) than in patients of TB without diabetes (52.75%) and without HIV (56.12%). Naranjo’s causality assessment accounted 47.12% of the ADRs as “probable”, 51.72% as “possible”, 1.15% as “doubtful”. WHO-UMC causality assessment scale accounted 43.68% as “probable”, 44.82% as “possible”, 8.05% as “unlikely”, and 3.44% as “unclassified”. Modified Hartwig and Siegel Scale for assessment of severity of ADRs showed 70.11% as mild, 27.58% as moderate and 2.29% as severe. Halla’s avoidability assessment scale showed 44.83% ADRs as ‘not avoidable’, 48.28% as ‘possibly avoidable’, 4.59% as ‘not evaluable’, and 2.29% as ‘definitely avoidable’.
Conclusion
Regular and vigilant drug monitoring of various ADRs can improve the clinical outcome, quality of life as well as patient adherence towards treatment. Results of this study can help to promote safer drug use and increase awareness regarding these ADRs. Data obtained can be used as a guide to make future decisions regarding therapy and risk benefit assessment of drugs in therapy.
Anti tubercular drugs, Drug monitoring, Tuberculosis
Introduction
Tuberculosis (TB) is a highly infectious chronic bacterial disease and is one of the top 10 leading causes of mortality and morbidity worldwide. Among the developing countries, India has the highest burden of TB. According to WHO Global TB Report, 2015, out of 9.6 million TB cases occurring globally, estimated incidence of TB in India is 2.2 million [1]. While in 2017 Global TB Report incidence of TB in India accounts for 28,00,000 which is about a quarter of the world’s TB cases [2].
National Tuberculosis Program was started by Government of India in 1962, while its pilot project RNTCP in 1993 and Directly Observed Treatment Shortcourse (DOTS) strategy was officially launched as RNTCP strategy in 1997 which by 2006 expanded its roots nationwide. DOTS has been started and proved to be the most cost effective and efficacious approach in controlling TB burden in India [3].
ATT drugs used for the treatment of TB are:
First line ATT drugs-Isoniazid, Rifampin, Pyrazinamide, Ethambutol, Streptomycin
Second line ATT drugs-Ethionamide, Prothionamide, Cycloserine, Terizidone, Para aminosalicylic acid, Rifabutin, Thioacetazone, Fluoroquinolones such as moxifloxacin, levofloxacin and gatifloxacin, Aminoglycosides such as kanamycin, amikacin, capreomycin.
DOTS involved treatment with first line ATT drugs-Isoniazid (INH), Rifampicin (Rif), Pyrazinamide (PZA) and Ethambutol (EMB). Some of the important and common adverse effects of these drugs are:
Isoniazid-Hepatitis, peripheral neuropathy, rashes.
Rifampicin-Orange urine/body fluids (sweat), flu-like syndrome, hepatitis.
Pyrazinamide-Hepatitis, hyperuricaemia, arthralgia, rashes.
Ethambutol-Optic neuritis (red-green color blindness), hyperuricaemia.
Streptomycin-Ototoxic effects, generally manifesting as dizziness, vertigo.
ADRs is defined as “Any harmful or unpleasant response due to the use of drug at therapeutic doses for the prevention or treatment of disease but can cause hazard from future administration and warrants alteration of the dosage regimen, or withdrawal of the product” [4]. So, by keeping constant watch on the ADRs will not only help the healthcare professionals to report them on time but also help to reduce the mortality due to these ADRs [5].
Around 6% of hospital admissions are estimated to be due to ADRs and about 6-15% of hospitalised patients experience serious ADRs imposing both physical and economic burden on the patient [6,7].
TB is a chronic debilitating infectious condition caused by Mycobacterium tuberculosis. It has been shown to be associated with various conditions like anaemia, diabetes mellitus, hypertension, HIV, hypothyroidism etc. Many researchers have found a bidirectional association between TB and DM. In developing countries where TB is endemic and burden of diabetes is increasing, this link of DM and TB seems to be more prominent there. As TB is a serious health threat, becomes more dangerous when associated with HIV and also found to be one of the leading causes of death.
There are similar studies describing the demographic profile of patients suffering from ADRs, distribution of ADRs regarding the treatment categories and predominant site of tubercular involvement [8]. The present study focused on the predisposing factors, causality assessment, severity assessment and avoidability assessment of the ADRs of first line ATT used under DOTS regimen which have not been dealt in earlier studies therefore, to know the further details of ADRs related to above described factors the study becomes relevant.
Materials and Methods
The present prospective observational analytical study was done at the Department of Respiratory Medicine, King George’s Medical University, Lucknow, Uttar Pradesh from May 2016 to April 2017. Total 115 patients were undertaken in the study. The study was started after ethical approval from the Institutional Ethics Committee-IEC number-81st ECM II-B Thesis/P29 KGMU Lucknow. Patients with proven TB who were kept on DOTS regimen under RNTCP, and those who gave written informed consent, satisfying the inclusion and exclusion criteria were recruited for the study. In this study we have attempted to identify the various predisposing factors, causality assessment, severity grading and avoidability of ADRs of first line ATT drugs.
Inclusion and exclusion criteria are as follows:
Inclusion Criteria
New diagnosed cases of pulmonary TB.
Patients of either sex.
Age more than 18 years.
Patients with normal baseline parameters such as normal liver function tests, kidney function tests, thyroid function tests, chest X-ray, blood sugar level (fasting and post-prandial) and HIV seropositivity.
No associated co-morbidities except HIV and Diabetes Mellitus.
Exclusion Criteria
Those who were unwilling to participate in the study.
Those who were unable to give consent in the study.
Those who were unable to give interview in the study.
Those with incomplete medical records.
Patients with chronic liver disease such as cirrhosis, chronic hepatitis and acute viral hepatitis.
Concurrent major psychiatric illness and/or concurrent major medical illnesses.
Patients with baseline parameters showing abnormal laboratory values.
Patients with end stage disease or severely ill patients.
This was an observational study and the procedure followed was in agreement with the ethical standards of the authority committee on human experimentation (Institutional or national). Diagnosis of TB was confirmed by sputum smear examination before enrolling the patient. The treatment was initiated as per category I-newly diagnosed patients of TB or Category II-previously treated patients pending drug sensitivity testing result (According to Treatment of TB: Guidelines, 4th edition, 2010, WHO, Geneva) [9]. Other baseline investigations had been done before starting DOTS regimen. For easy follow-up, each patient were allotted a unique patient identification number. First line ATT drugs like Isoniazid, Rifampicin, Pyrazinamide, Ethambutol and Streptomycin were given during intensive and continuous phase. Patients were given the following instructions:
They were instructed not to stop the ATT medications themselves without consulting the physician as complete course of therapy is mandatory.
During the course of treatment discolouration of urine, can occur and one should not be bothered by that.
Patients were instructed to take the medication empty stomach (rifampicin).
For any vision defects consult the treating physician.
Intake of proper and nutritious diet is must.
If any drug reaction occurs after consuming the drug immediately contact your doctor.
In further visits, patients were monitored for any ADRs or fresh complaints. Causality is the relationship between undergoing drug therapy and the ADR.
Causality assessments were carried out using:
Naranjo’s algorithm causality assessment scale [10]:
Total scores range from -4 to +13; the reaction is considered:
Definite, score- 9 or higher
Probable, score- 5 to 8
Possible, score- 1 to 4
Doubtful, score- 0 or less.
WHO-UMC causality assessment [11]: WHO-UMC classification of ADRs into following categories on the basis of causality assessment:
Certain✓ Any abnormal laboratory test or clinical event and its reasonable relationship with administration of drug.
✓ Not explainable by concurrent disease or drugs/chemicals.
✓ Dechallenge (withdrawal of drug) should be possible and clinically relevant both pharmacologically and physiologically.
✓ Rechallenge information should be satisfactory.
Probable/likely✓ Any abnormal laboratory test or clinical event and its reasonable relationship with administration of drug.
✓ Unlikely to be explained by concurrent diseases or drugs/chemicals.
✓ Dechallenge-clinically reasonable response.
✓ Rechallenge information not required.
Possible✓ Any abnormal laboratory test or clinical event and its reasonable relationship with administration of drug.
✓ Can also be explained by the ongoing disease or other drugs/chemicals.
✓ Dechallenge-lacking or unclear.
Unlikely✓ Any abnormal laboratory test or clinical event and its improbable relationship with administration of drug.
✓ Underlying diseases or drugs can provide a possible explanation.
Conditional/Unclassified✓ Any abnormal laboratory test or clinical event reported as an adverse effect.
✓ More data needed for proper assessment.
✓ Additional data under examination.
Unassessable/Unclassifiable✓ Report as an adverse reaction.
✓ Information-insufficient or contradictory so report cannot be judged.
Severity of ADRs was assessed using Modified Hartwig and Siegel scale [12] as:
Mild/Minor: No antidote, therapy or prolongation of hospitalisation is required.
Moderate: Here it requires changes in drug therapy, specific treatment, or an increase in hospitalisation at least by a day.
Severe: Potentially life threatening, causing permanent damage or requiring intensive medical care.
Avoidability of ADRs was assessed by using Halla’s avoidability scale as [13]:
Definitely avoidable-Any drug treatment differing with the present day knowledge of good medical practice resulted into an untoward event, taking into account the known circumstances.
Possibly avoidable-The prescription was not incorrect, but the event could have been avoided by an effort, exceeding the obligatory demands.
Not avoidable-The event could not have been avoided by any responsible means, or was an unpredictable event in the course of a treatment fully in accordance with good medical practice.
Unevaluable-Conflicting evidence or data could not be obtained.
Statistical Analysis
Categorical variables were presented in number and percentage (%). Qualitative variables were compared using Chi-Square test/Fisher’s-exact test as appropriate. A p-value of <0.05 was considered statistically significant. The data was entered in MS EXCEL spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
Results
A total of 115 patients were recruited for the study. Number of males were 66 (57.39%), while females were 49 (42.61%) of age group 18-80 years and above. Most of the patients fall in the age group 41-50 (26.09%) followed by 31-40 (20.00%). The number of ADRs was more in patients of TB with DM than in patients of TB without DM [Table/Fig-1]. The number of ADRs was more in patients of TB with HIV than in patients of TB without HIV [Table/Fig-2].
Showing the association of ADRs in patients of TB with DM and without DM.
| ADR | TB with DM | TM without DM |
|---|
| Present | 19 (79.16) | 48 (52.75) |
| Absent | 5 (20.84) | 43 (47.25) |
| Total | 24 (100) | 91 (100) |
Showing the association of ADRs in patients of TB with HIV and without HIV.
| ADR | TB with HIV | TB without HIV |
|---|
| Present | 12 (70.59) | 55 (56.12) |
| Absent | 5 (29.41) | 43 (43.88) |
| Total | 17 (100) | 98 (100) |
The various predisposing factors, causality, severity and avoidability of adverse drug reactions are described below:
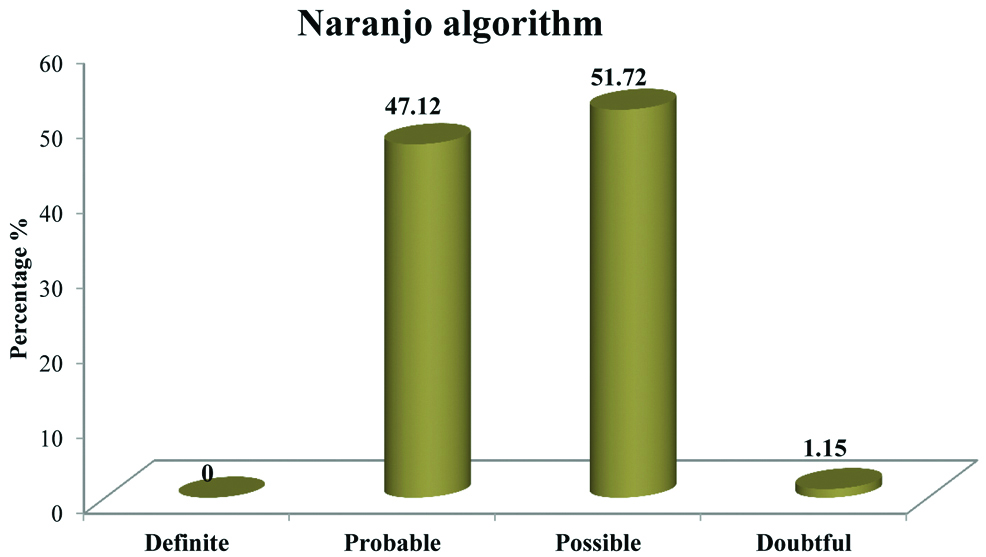
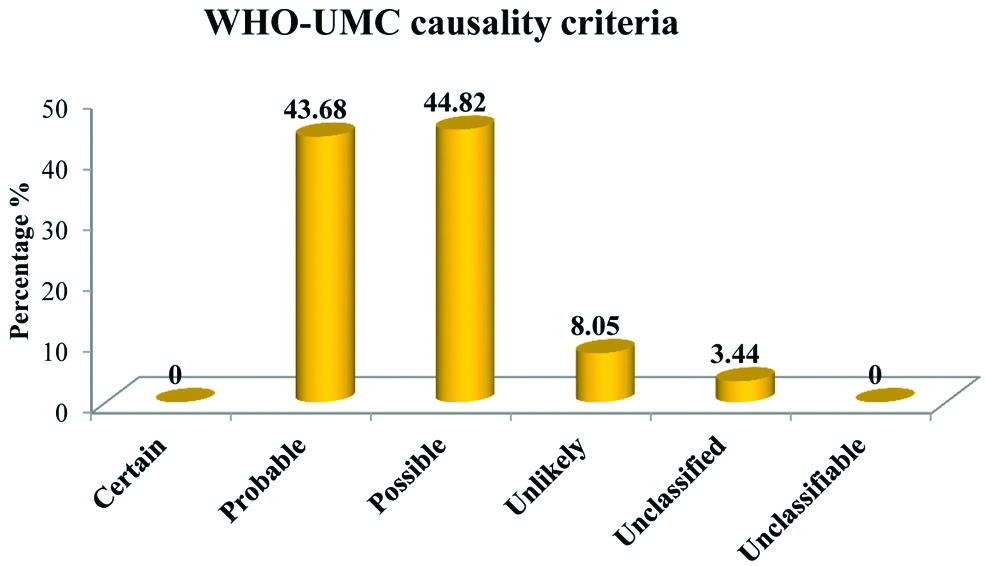
According to Naranjo’s algorithm and WHO-UMC scale for causality assessment of ADRs, most of the ADRs fall under ‘possible’ category [Table/Fig-3,4].
Pattern of distribution of causality assessment according to Naranjo’s scale.
Pattern of distribution of causality assessment according to WHO-UMC scale.
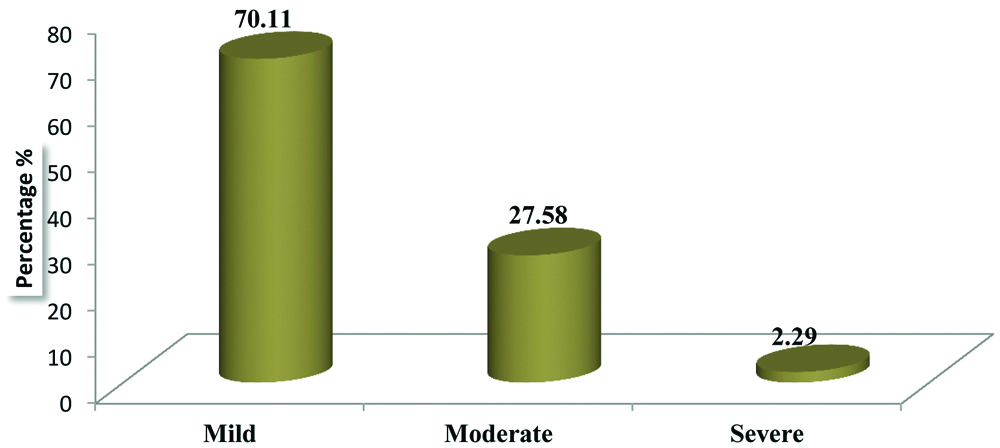
According to modified Hartwig and siegel scale for severity assessment of ADRs, most of the ADRs was of ‘mild grade’ [Table/Fig-5].
Severity assessment of ADRs through Hartwig’s scale.
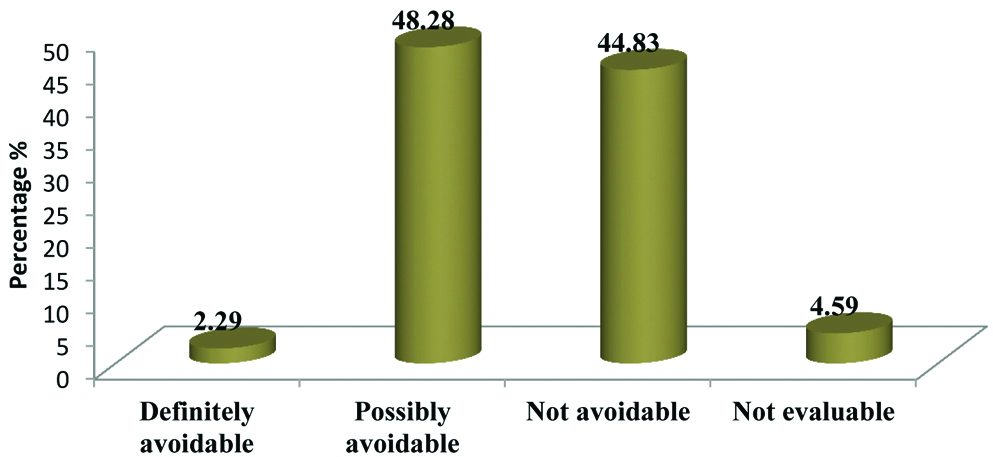
According to Halla’s avoidability scale most of the ADRs were ‘possibly avoidable’ [Table/Fig-6].
Categorization of ADRs based on Halla’s avoidability scale.
Discussion
Tuberculosis (TB) is one of the leading infectious disease causing widespread morbidity and mortality as well as tremendous health and economic burden on the community. For an effective control and management of the rapidly growing disease strict adherence to the anti-TB regimen is needed. To enhance this compliance DOTS has indisputably played a competent role [14]. The present study focuses on ADR reporting of first line anti-tubercular drugs in patients who are kept on DOTS strategy. Usually, these drugs are well tolerated but most often they get associated with untoward effects. Serious drug reactions from any of the first line ATT lead to withdrawal or refusal of treatment thus increasing resistance and affecting the adherence towards the treatment [15]. Study related to incidence and pattern of ADRs by Tutu S et al., highlights the gravity of ADRs in patients treated for TB under DOTS [8]. Number of males were more 66 (57.39%) as compared to females 49 (42.61%). Most of the patients fall in the age group 41-50 (26.09%) followed by 31-40 (20.00%). ADRs was more in patients of TB with DM (79.16%) than in patients of TB without DM (52.75%). A similar incidence of ADRs has been reported in the study conducted by Hire R et al., which observed that in TB patients with DM, incidence of ADRs was 80% and in TB patients without DM it was 47% [16]. Incidence of ADRs was more in patients with HIV (70.59%) than in patients without HIV (56.12%). In our study causality assessment using Naranjo’s scale have shown that majority of ADRs were under category “possible” (51.72%) followed by “probable” (47.12%) while assessment through WHO-UMC scale shows that maximum number of ADRs fell under “possible” (44.82%) followed by “probable” (43.68%). On the similar line study conducted by Anusha N et al., showed that majority of ADRs (87.5%) had a “possible” relationship and only 12.5% of the ADRs had a “probable” relationship with the suspected drugs [17], while Gholami K et al., found that probable reaction was little more than possible [18]. Hartwig scale for severity assessment of ADRs reflects that majority of ADRs were “mild” (70.11%) and “moderate” (27.58%). This finding gets supported by a study conducted by Chhetri AK et al., reported 93.33% of ADRs were of mild level while 6.66% were of moderate level [19]. Avoidability scale for ADRs shows that a bigger proportion of ADRs belonged to “possibly avoidable” (48.28%) followed by “not avoidable” (44.83%). This goes in tune with the study conducted by Hire R et al., where 33.33% were of “not avoidable” category while 54.16% belonged to “possibly avoidable” category [16]. From the above discussion it has been suggested that ADRs becomes more frequent when TB gets associated with other condition such as DM and HIV. Findings were found to be consistent with the previously conducted studies on ADRs due to ATT. These findings will help in reducing mortality and morbidity due to ADRs as well as increasing compliance and quality of life of the patient. This can be attained by strengthening the healthcare support system and creating awareness among the general public by educating them.
Limitation
Short duration of study i.e., one year and small sample size were the major limitations of the study though the value of its result cannot be ignored. Points regarding the causality evaluation of potential drug interactions were not addressed in Naranjo scale. Dechallenging and rechallenging was not done in order to establish the causative agent. A problem of recall bias may have crept in the study which can’t be denied straight forward.
The study could have been improved by taking larger sample size along with longer follow-up period could have provided with better power of the study and better rate of incidence database for TB drug regimen associated ADRs.
Conclusion
TB patients along with co-morbid conditions such as DM, HIV etc., increase the individuals’ susceptibility to ADRs. ADRs have been associated with clinical morbidity and may lead to even mortality. Despite the fact that most cases have been treatable, the problem is persistent mainly due to a high attrition rate that is correlated with ADR mediated complications. So, there is an urgent need for regular monitoring, timely reporting, adequate management of such drug related ADRs for increasing patient compliance and decreasing morbidity to improve clinical outcome in TB and quality of life of patients. All these can be attained by strengthening the healthcare system, educating health care professionals and general public regarding the importance of adherence to treatment, sensitising the community regarding the disease and its hazards, updating knowledge of the ADRs, its vigilant reporting and means to avoid its occurrence, need of active and passive surveillance, importance of hygiene in order to control the disease.
[1]. Central tuberculosis division (2015). TB India 2015 [Internet]. Revised National TB control programme. Annual status report. New Delhi: Central tuberculosis division. [cited 2018 Dec 15]. Available from https://tbcindia.gov.in/showfile.php?lid=3166 [Google Scholar] [CrossRef]
[2]. Central tuberculosis division (2018). India TB Report 2018 [Internet]. Revised National TB control programme. Annual status report. New Delhi: Central tuberculosis division. [cited 2018 Dec 15]. Available from https://tbcindia.gov.in/showfile.php?lid=3314 [Google Scholar]
[3]. National Health Portal. Tuberculosis management 2018, Jul 12 [Internet], National Health Portal India. [cited 2019 Mar 09]. Available from https://www.nhp.gov.in/disease/respiratory/lungs/tuberculosis [Google Scholar]
[4]. Edwards IR, Aronson JK, Adverse drug reactions: definitions, diagnosis, and managementThe Lancet 2000 356(9237):1255-59.10.1016/S0140-6736(00)02799-9 [Google Scholar] [CrossRef]
[5]. ICH Topic E2E Pharmacovigilance Planning (Pvp) [Internet], Note for guidance on planning pharmacovigilance activities (CPMP/ICH/5716/03) June 2005. [cited 2018 Dec 15]. Available from https://www.ema.europa.eu/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-25.pdf [Google Scholar]
[6]. Einarson TR, Drug-related hospital admissionsAnnals of Pharmacotherapy 1993 27(7-8):832-40.10.1177/106002809302700702 [Google Scholar] [CrossRef]
[7]. Jose J, Rao GM, Pattern of adverse drug reactions notified by spontaneous reporting in an Indian tertiary care teaching hospitalPharmacol Res 2006 54:226-33.Available from https://www.ncbi.nlm.nih.gov/pubmed/1678116310.1016/j.phrs.2006.05.00316781163 [Google Scholar] [CrossRef] [PubMed]
[8]. Tutu S, Kant S, Verma AK, Sachan AK, Nath R, Kumar N, Incidence and pattern of adverse drug reactions (ADRs) in patients treated for tuberculosis under DOTS at a tertiary care hospital of Northern IndiaInt J Pharm Sci & Res 2018 9(11):4950-55.10.13040/IJPSR.0975-8232.9(11).4950-55 [Google Scholar] [CrossRef]
[9]. World Health Organization & World Health Organization. (2010). Treatment of tuberculosis: guidelines, 4th edition. Geneva: World Health Organization [Google Scholar]
[10]. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, A method for estimating the probability of adverse drug reactionsClin Pharmacol Ther 1981 30(2):239-45.Available from https://ascpt.onlinelibrary.wiley.com/doi/abs/10.1038/clpt.1981.15410.1038/clpt.1981.1547249508 [Google Scholar] [CrossRef] [PubMed]
[11]. The use of the WHO-UMC system for standardised case causality assessment-1-The use of the WHO-UMC system for standardised case causality assessment Why causality assessment? [Internet]. [cited 2019 Apr 18]. Available from: https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf [Google Scholar]
[12]. Hartwig SC, Siegel J, Schneider PJ, Preventability and severity assessment in reporting adverse drug reactionsAmerican Journal of Health-System Pharmacy 1992 49(9):2229-32.10.1093/ajhp/49.9.2229 [Google Scholar] [CrossRef]
[13]. Hallas J, Harvald B, Gram LF, Grodum E, Brøsen K, Haghfelt T, Drug related hospital admissions: The role of definitions and intensity of data collection, and the possibility of preventionJournal of Internal Medicine 1990 228(2):83-90.10.1111/j.1365-2796.1990.tb00199.x2394974 [Google Scholar] [CrossRef] [PubMed]
[14]. Rohit Jairus R, Determine the factors influencing dots compliance among tuberculosis patients of selected dots centers of Ludhiana, PunjabInternational Journal of Development Research 2017 7(7):13771-74.Available from https://www.journalijdr.com/sites/default/files/issue-pdf/9368.pdf [Google Scholar]
[15]. Xiaozhen Lv, Tang S, Xia Y, Wang X, Yuan Y, Hu D, Adverse reactions due to Directly Observed Treatment Strategy therapy in Chinese tuberculosis patients: A prospective studyPLOS ONE 2013 8(6):1-7.Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3672195/pdf/pone.0065037.pdf10.1371/journal.pone.006503723750225 [Google Scholar] [CrossRef] [PubMed]
[16]. Hire R, Kale AS, Dakhale GN, Gaikwad N, A prospective, observational study of adverse reactions to drug regimen for multi-drug resistant pulmonary tuberculosis in central IndiaMediterr J Hematol Infect Dis 2014 6(1)Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4165500/10.4084/mjhid.2014.06125237474 [Google Scholar] [CrossRef] [PubMed]
[17]. Anusha N, Topno I, Purty AJ, Adverse drug reactions monitoring among TB patients on anti-tubercular drugs under RNTCP in PondicherryInt J Adv Res 2014 2(12):165-73. [Google Scholar]
[18]. Gholami K, Kamali E, Hajiabdolbagh M, Shalviri G, Evaluation of anti-tuberculosis induced adverse reactions in hospitalized patientsPharm Pract 2006 4:134-38. [Google Scholar]
[19]. Chhetri AK, Saha A, Verma SC, Palaian S, Mishra P, A study of adverse drug reactions caused by first line anti-tubercular drugs used in Directly Observed Treatment, Short course (DOTS) therapy in western Nepal, PokharaJ Pak Med Assoc 2008 58(10):531-36. [Google Scholar]