Caudal block is a reliable and safe technique in paediatric patients. It reduces the anaesthetic agent requirement, attenuates surgical stress and provides better postoperative analgesia.
The drawback of caudal block is the short duration of action after a single injection [1]. Ultrasonography-guided caudal block has gained popularity because of its lower complication rates [2,3]. Variety of adjuvants, such as epinephrine, opioid, and α2 agonists have been used to prolong the duration of caudal analgesia [4,5].
The present authors hypothesised that dexmedetomidine in a dose of 1 μg/kg, as an adjuvant to ropivacaine 0.25% (1 mL/kg) in caudal block would provide prolonged postoperative analgesia and reduced pain scores with minimal adverse effects in paediatric patients.
This randomised, double-blinded, placebo-controlled study was designed to compare the analgesic effect of caudal ropivacaine with dexmedetomidine versus ropivacaine in paediatric patients undergoing infra-umbilical surgeries.
Materials and Methods
This was a double-blinded randomised study, conducted at a tertiary care institution. This study was conducted after getting clearance from the Institutional Ethical Committee (AIMS/IEC/2180/2017-2018) and obtaining written informed consent from guardians of patients. The study was conducted from January 2018 to December 2018. Sixty patients, belonging to the American Society of Anaesthesiologist (ASA) Classes 1 and 2, in the age group of 2-12 years, of either gender, who underwent infra-umbilical surgery, were enrolled [Table/Fig-1].
Patients with history of developmental delay, mental retardation and known allergy to study drugs, injection site infection, neurological disease, coagulopathies, spinal deformities and parental refusal were excluded from the study.
Patient’s age, weight, and baseline vital parameters were recorded, detailed history and examinations were done at pre anaesthestic visit. All patients were kept nil per orally as per standard protocol (2 hours for clear liquid and 6 hours for semisolid and solid food).
Randomisation was done with a computer-generated list and children were equally assigned into two groups R (ropivacaine) and RD (ropivacaine plus dexmedetomidine), with 30 members in each group. An anaesthesiologist not involved in the study prepared the study of drugs.
Upon arrival of patient in the operation theatre, ASA standard monitor was connected and baseline parameters such as Heart Rate (HR), Blood Pressure (BP), Oxygen Saturation (SpO2), and Respiratory Rate (RR) were recorded. Patients were induced with 8% sevoflurane in oxygen with spontaneous ventilation, IV line was secured with either 22 or 24 gauge cannula, fluid was infused as calculated and an appropriate sized Laryngeal Mask Airway (LMA) was inserted. After the LMA was inserted, sevoflurane concentration was reduced to 2%; patients were placed in left lateral position and with aseptic precautions, ultrasound-guided caudal block was administered under real-time guidance with a high frequency (5-10 MHz) ultrasound probe (Micromaxx™ Sonosite, Inc., Bothell, WA98021, USA). An axial image for the sacral hiatus and dorsal sacrococcygeal ligament between the two sacral cornua were obtained and then transducer was rotated by around 90° to obtain the sagittal view of the sacral hiatus. With real-time guidance, a 23-gauge, 5 cm needle was introduced and advanced at around 45° angulation until it pierced the dorsal sacrococcygeal ligament. After careful negative aspiration for blood and CSF, study drug was injected.
Group R received 0.25% ropivacaine 1 mL/kg+0.5 mL normal saline.
Group RD received 0.25% ropivacaine 1 mL/kg+1 μg/kg dexmedetomidine (in 0.5 mL volume).
After noting the injection time, patients were turned back to supine position. Anaesthesia was maintained using sevoflurane in oxygen/air (50:50) mixture. Intraoperatively, no other drugs like narcotics, analgesics, sedatives or anti-emetics were administered. After the surgery was completed, LMA was removed and the patients were transported to the Post Anaesthesia Care Unit.
The present authors assessed the onset of block by applying mechanical stimulus at surgical site after 5, 10 and 15 minutes of the injection. The successful onset of the block is defined as the time in minutes between injection of local anaesthetic and the absence of motor response or absence of >20% increase in HR on application of mechanical stimulation. Patients with patchy and failed caudal block were excluded from the study.
The present authors recorded vital parameters before induction, just before and after skin incision and every five minutes for half hour, and every 15 minutes for one hour. The present authors assessed the postoperative pain using the Face, Legs, Activity, Cry, Consolability (FLACC) pain scale. The present authors used Ramsay sedation scale to assess postoperative sedation. The duration of adequate caudal analgesia was recorded, and paracetamol syrup was administered as 10 mL/kg at FLACC pain score ≥4 and total dose of rescue analgesic administered in observation period was recorded.
Patients were monitored for adverse-effects such as nausea, vomiting, bradycardia, respiratory depression and hypotension and treated accordingly. The present authors calculated the sample size to be 30 in each group to establish a significant difference in the mean duration of analgesia and total analgesic doses requirement during first 24 hours in both groups with a α error of 0.05 [8].
Statistical Analysis
The data obtained from the present study was tabulated and analysed using the computer software (SPSS for Windows, Version 16.0. Chicago, SPSS Inc.). The quantitative data were compared using Student’s t-test. We used Mann-Whitney U-test for comparing skewed data. Qualitative or categorical data were expressed as frequencies and analysed with Chi-square or Fisher’s exact test. The p-value<0.05 was accepted as statistically significant.
Results
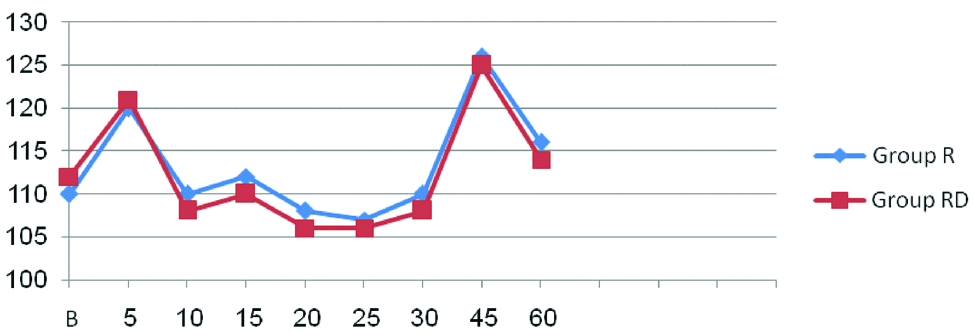
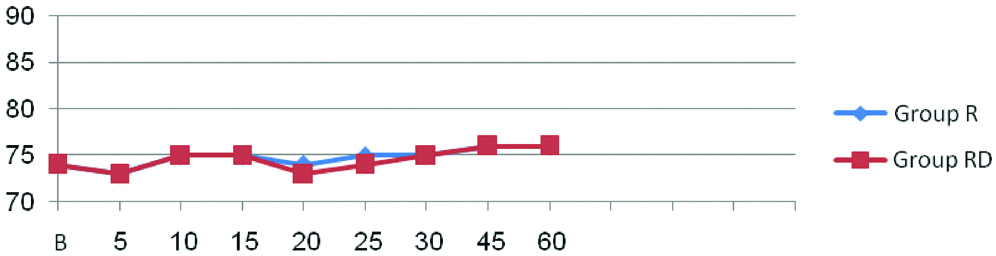
Demographic profile of patients and operative characteristics between both groups were similar [Table/Fig-2]. There were no significant differences in the heart rate [Table/Fig-3] and mean arterial pressure [Table/Fig-4] between the two groups at any time interval (p-value >0.05)
TDemographic profile of patients.
| Character | Group R (n=30) | Group RD (n=30) | p-value |
|---|
| Age (years) | 6.67±2.83 | 6.5±2.92 | 0.379 |
| Weight (kg) | 22.6±3.89 | 22±4.59 | 0.294 |
| Sex (m/f) | 28/2 | 29/1 | |
| Duration of surgery(min) | 53.40±9.53 | 53.09±12.26 | 0.458 |
Group R-Ropivacaine; Group RD-Ropivacaine+Dexmedetomidine, p-value >0.05; not significant, M/F- Male/Female
Comparison of Intraoperative heart rate between two groups.
Group R-Ropivacaine; Group RD-Ropivacaine+Dexmedetomidine, p-value >0.05; not significant
Comparison of intraoperative mean arterial pressure between two groups.
Group R-Ropivacaine; Group RD-Ropivacaine+Dexmedetomidine; p-value >0.05; not significant
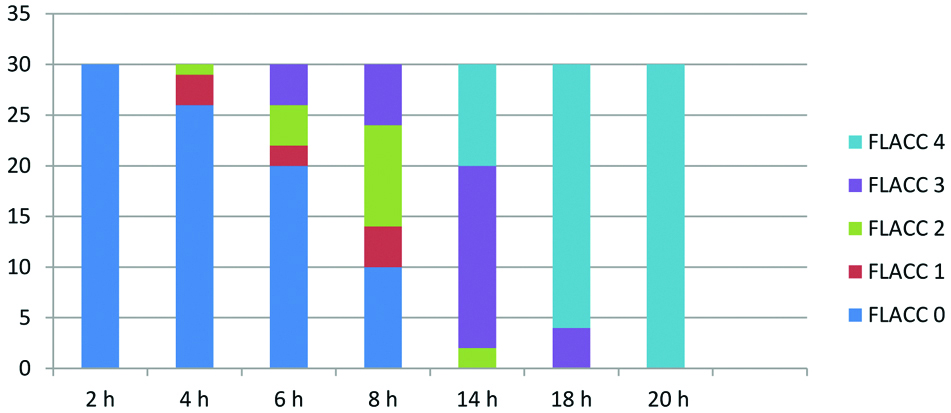
The mean duration of analgesia was longer in Group RD (790.77±7.70 minutes) as compared to Group R (377.97±12.20 minutes), p-value<0.0001 [Table/Fig-5]. The total number of doses of rescue analgesic required were lesser in Group RD as compared to Group R, p-value <0.05 [Table/Fig-5]. Group R patients demonstrated significantly higher FLACC score compared to Group RD patients, where 22 out of 30 children achieved a FLACC score of 4 at 6th hour compared with 0 patients in Group RD, whereas 26 out of 30 children of Group RD had FLACC score of 4 at 16th hour of postoperative period [Table/Fig-6,7]. The incidence of sedation, nausea, vomiting and the use of antiemetic medications were not statistically significant in either group [Table/Fig-8].
Duration of analgesia and rescue analgesic doses required in 24 hours.
| Parameter | Group R n (30) | Group RD n (30) | p-value |
|---|
| Duration of Analgesia | 377.97±12.20 min | 790.77±7.70 min | <0.001* |
| Number of doses of rescue analgesic required | n (%) | n (%) | |
| 1 dose | 2 (6.67%) | 27 (90%) | |
| 2 dose | 9 (30%) | 3 (10%) | |
| 3 dose | 19 (63.33%) | | |
*p-value<0.001; highly significant, Values expressed as Mean±SD and number (percentage); Group R-Ropivacaine; Group RD-Ropivacaine+Dexmedetomidine
FLACC score of group R.
h-Hour, y axis-number of patients
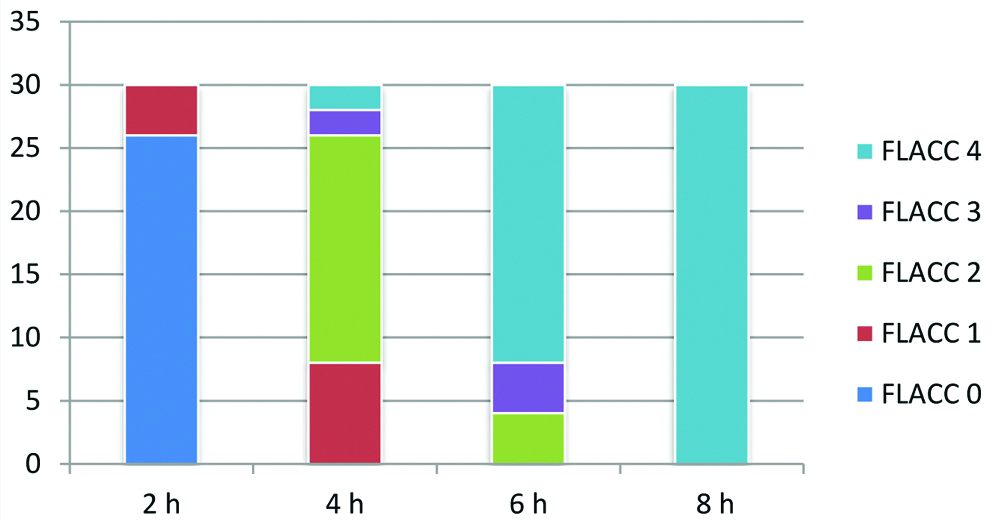
FLACC score of group RD.
h-hour, y axis- number of patients
Ramsay sedation score during postoperative period.
| Time | Group R | Group RD |
|---|
| End of surgery | 3 (2-3) | 3 (2-3) |
| 1 hour | 1 (0-2) | 1 (0-2) |
| 2 hour | 1 (0-2) | 1 (0-2) |
| 3 hour | 1 (0-2) | 1 (0-2) |
| 4 hour | 0 (0-0) | 0 (0-0) |
Group R-Ropivacaine; Group RD-Ropivacaine+Dexmedetomidine
Values expressed as median (range)
Discussion
In the present study, the present authors administered dexmedetomidine 1 μg/kg as an adjuvant to ropivacaine 0.25% (1 mL/kg) as a single shot caudal block and observed that the duration of postoperative analgesia (FLACC <4) without any rescue, analgesic was significantly longer in Group RD than Group R. In support of the present study, Jain K et al., also administered dexmedetomidine in a dose of 1 μg/kg, as an adjuvant with 0.25% ropivacaine caudally, and concluded that the duration of postoperative analgesia was significantly longer in the group receiving ropivacaine-dexmedetomidine mixture than the group receiving ropivacaine alone [9].
Similarly, Anand VG et al., utilised dexmedetomidine and clonidine, in doses of 2 μg/kg, as adjuvant with 0.25% bupivacaine in caudal blocks [10]. They concluded that the duration of analgesia was significantly prolonged in the group receiving bupivacaine with dexmedetomidine or bupivacaine-clonidine mixture compared to the group receiving only bupivacaine. Neogi M et al., compared clonidine 1 μg/kg and dexmedetomidine 1 μg/kg as adjuvants to ropivacaine 0.25% in caudal block in paediatric patients and concluded that mean (SD) duration of analgesia was 15.26 hours in dexmedetomidine group, which was significantly prolonged as compared to clonidine group 13.17 hours and ropivacaine group 6.32 H [11].
In the present study, we found that the mean time to first rescue analgesic demand was significantly prolonged and cumulative doses of rescue analgesic obtained during the observation period was significantly less in Group RD as compared to Group R. In support of the present study, Saadawy I et al., also found in their study that 77% of the children in the Bupivacaine-Dexmedetomidine (BD) group versus 10% in group bupivacaine did not require additional analgesia and cumulative postoperative analgesic requirements were significantly less in the BD group (p-value <0.01) during the first 24 hours postoperative period [12].
The pre, intra, and postoperative haemodynamic variables [Table/Fig-2,3] between the groups R and RD were comparable and were statistically insignificant and none of them required therapeutic interventions. There were no episodes of clinically significant postoperative complications such as postoperative nausea and vomiting, respiratory depression, urinary retention, pruritus, hypotension, and bradycardia in any of the groups. The results of the present study depicts that in addition to prolonged postoperative analgesia, dexmedetomidine has a favourable safety profile and stable haemodynamics, which are supported by the reports published by many other authors [13-16].
Limitation
The present study lacked a group with IV dexmedetomidine. Hence, we could not comment on the potential local effect of dexmedetomidine. Therefore, there is a need of future studies with IV dexmedetomidine as a control group to evaluate potential local effects of dexmedetomidine.
Conclusion
The present results allow us to conclude that addition of dexmedetomidine (1 μg/kg) to caudal ropivacaine 0.25% at 1 mL/kg significantly prolonged postoperative analgesia in children undergoing infra-umbilical surgeries without notable side effects. Hence, dexmedetomidine may be used as a safe and effective adjuvant for caudal analgesia in paediatric patients.
Group R-Ropivacaine; Group RD-Ropivacaine+Dexmedetomidine, p-value >0.05; not significant, M/F- Male/Female
*p-value<0.001; highly significant, Values expressed as Mean±SD and number (percentage); Group R-Ropivacaine; Group RD-Ropivacaine+Dexmedetomidine
Group R-Ropivacaine; Group RD-Ropivacaine+Dexmedetomidine
Values expressed as median (range)