Hirsutism is defined as excessive growth of terminal hair in women in male distribution pattern in areas like face, chest, abdomen, upper thigh, and areola [1]. It is seen in almost 5-10% of women and may be related to significant underlying endocrine disorders like PCOS, CAH, hypothyroidism and Cushing’s syndrome.
Women having hirsutism have a negative impact on quality of life. The presence of PCOS is associated with greater impairment of quality of life [2]. They suffer from social phobia, insecurity about interpersonal relationships, shattered confidence and profound psychological sequelae with impaired self-image of the patient’s feminine identity [2,3].
Hirsutism can be either androgen induced or non-androgen induced. Excessive endogenous androgen production which can be ovarian or adrenal and consumption of exogenous androgenic drugs can lead to androgen induced type, while non-androgen induced can be due to idiopathic or familial causes [4]. In 95% of cases, hirsutism is secondary to benign conditions like IH or PCOS, other causes being CAH, Cushing’s syndrome, and benign and malignant androgen-secreting adrenal or ovarian tumours [5]. The underlying pathophysiology is believed to be due to exaggerated activity of 5α reductase, androgen receptor polymorphisms, and altered androgen metabolism that leads to a state of hyperandrogenism [6]. Hyperandrogenism with hirsutism can be associated with various signs and symptoms such as Acanthosis Nigricans (AN), obesity, acne, virilisation, Female Pattern Hair Loss (FPHL) and pelvic mass [7]. Virilisation means a condition in which women develop male-pattern hair growth and other masculine features like deepening of voice and increased muscle mass along with clitoromegaly. Presence of virilisation indicates hyperandrogenism secondary to excessive endogenous androgen production (ovarian/adrenal) or exogenous androgenic drugs. The Ferriman-Gallwey-Score, established in 1961 is a method of evaluating and quantifying hirsutism [8]. It has been identified both PCOS and IH as common causes of hirsutism. However, there is paucity of published literature regarding the incidence of HAIR-AN syndrome in hirsutism patients. Thus, due to its varied aetiology and associations, this study was carried out to find out the most common cause of hirsutism and assess the various associated hormonal and metabolic abnormalities including HAIR-AN syndrome. Also, to the best of our knowledge, there are no studies on hirsutism published from central part of India.
Materials and Methods
This was a retrospective cross-sectional study in which medical records of patients having hirsutism from prefilled proformas, attending the outpatient clinic of Department of Dermato-Venereo-Leprology, during June 2016 to May 2018 (2 years duration) were analysed.
All patients of hirsutism in the reproductive age group (15 to 45 years) who attended the outpatient department during the period of study with modified Ferriman-Gallwey (mF-G) score of eight or more were included in the study which amounted to sample size of 50. Pregnant or Lactating women, patients who had received oral contraceptive pills or/and other anti-androgen drugs in previous three months, patients who had received drugs known to cause hirsutism or interfere with the hormonal studies and those patients with mF-G score less than eight were excluded from the study.
Informed patient consent was obtained from all patients. Institutional Ethical Clearance (IEC) approval was obtained. (Number: 1402 EC/Pharmac/GMC/NGP/).
The mF-G score used to determine the severity of hirsutism is calculated by assessing the extent of hair growth in nine key anatomical sites i.e., the upper lip, chin, areola and chest, upper back, lower back, upper abdomen, lower abdomen, thighs, and upper arms [3,4]. The growth of hair is graded from grade 1 to 4 based on the extent of area involved at each of the nine anatomical sites [Table/Fig-1]. Final score is the addition of grade at each site. The total score ranges from 0 to 36. Based on mF-G score, severity was graded as mild (score 8-16), moderate (score17-24), and severe (score >24) [3].
Modified ferriman-gallwey semiquantitative scoring for hirsutism [8].
| Site | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|
| Upper lip | Few hair at outer margin | Small moustache at outer margin | Moustache from halfway to outer margin | Moustache upto midline |
| Chin | Few scattered hair | Scattered hair with small concentration | Complete but light cover | Complete and heavy cover |
| Chest | Circumareolar hair | Circumareolar and midline hair | Fusion of circumareolar and midline hair giving three quarter cover | Complete cover |
| Upper back | Few scattered hair | Scattered hair with small concentration | Complete but light cover | Complete and heavy cover |
| Lower back | Sacral tuft of hair | Sacral tuft with lateral extension | Three quarter cover | Complete cover |
| Upper abdomen | Few midline hair | More concentration of midline hair | Half cover | Complete cover |
| Lower abdomen | Few midline hair | Midline streak of hair | Midline band of hair | Inverted V shaped growth |
| Upper arm and thigh | Sparse hair covering less than quarter of limb | Coverage of more than a quarter but still incomplete | Complete but light cover | Complete and heavy cover |
| Forearms and legs | - | - | - | Complete and heavy cover |
Medical records of the patients were analysed for history that included the duration of symptoms, age, height, weight, BMI, WC and mode of onset of symptoms, age at menarche and the presence of menstrual irregularity. The WC was measured with circumference measurement tape in cm at a point midway between the iliac crest and the costal margin (lower rib) and according to the modified NCEP ATP III (National Cholesterol Education Program’s Adult Treatment Panel III) criteria, the cut-off points for WC for women of Asian origin was 80 cm [9]. The menstrual patterns were defined as regular cycles if the length of cycle was between twenty-one and thirty-five days. The cycle was considered as irregular if the patient had oligomenorrhea (bleeding at interval greater than thirty-five days), polymenorrhea (bleeding at intervals of less than twenty-one days), or amenorrhea (absence of menstruation for twelve months or more). A history of weight gain or drug intake was noted. A careful family history of hirsutism, infertility, and other metabolic disorders was also obtained from records of every patient. BMI was calculated in all patients and patients with BMI ≥25 kg/m2 were labelled as overweight and those with BMI ≥30 kg/m2 were labelled as obese [3]. History of signs of virilisation that included acne, clitoromegaly, deepening of voice and increased muscle mass were noted. The patients were examined for presence of FPHL and AN. For AN, patients were examined with respect to dark coarse thickened skin with velvety texture in symmetrical distribution over nape of neck, axillae and groins. Details of complete systemic examination (including thyroid, breast, and pelvic) were also noted in these patients.
Records of hormonal assays were assessed. Blood sampling was done in the early follicular phase of menstrual cycle (day 3 to 4) after overnight fasting for 10 to 12 hours and was collected around 8-9 am. Serum FSH, serum LH, prolactin, serum Free (F), serum Dehydroepiandrosterone Sulphate (DHEAS), 17-hydroxyprogesterone (17-OHP), lipid profile (Serum triglycerides, total cholesterol, LDL & HDL cholesterol), thyroid profile (Serum TSH, T3 & T4), fasting blood sugar, serum fasting insulin were assayed. Transabdominal USG was done in all patients during the follicular phase of menstrual cycle (Day 5-7). The USG criteria to define polycystic ovaries were presence of twelve or more follicles in each ovary measuring two to nine mm in diameter, and/or increase in ovarian volume (>10 mL) and only one ovary fitting this definition is sufficient for diagnosis [10]. Presence of regular ovulation and normal hormonal profile were diagnostic of IH, whereas PCOS was diagnosed on the basis of Rotterdam Criteria 2003, in which two of the following three criteria are required:
Presence of oligo and/or anovulation;
Features of Hyperandrogenism:
Clinical (hirsutism, acne, male pattern alopecia, AN) or
Biochemical (raised free androgen index or free testosterone);
Polycystic ovaries on ultrasound with ≥12 follicles (2-9 mm diameter) or ovarian volume >10 cc [10].
HAIR-AN syndrome was diagnosed when patient had hyperandrogenism (clinically or biochemically), insulin resistance (HOMA-IR index >2) and clinically evident AN [11]. HOMA-IR was calculated using the formula: fasting insulin x fasting glucose/405 [11]. Hirsutism secondary to hypothyroidism was diagnosed when all investigations were within normal limit except for decreased serum TSH.
Statistical Analysis
Statistical analysis was done using STATA version 14.0 software. Chi-square test was used for comparison between categorical variables (viz., acne and menstrual history) One-way ANOVA (Analysis of variance) was used for comparison of means of WC, BMI, mF-G score and hormone profile in different aetiologies of hirsutism for normalised data and Kruskal-Wallis ANOVA test was used for non-normalised data. Multiple comparisons were performed by Bonferroni t-test to compare any type of significant difference of study parameters between two aetiologies. The p-value of <0.05 was considered significant. The p-value of <0.001 was considered as highly significant.
Results
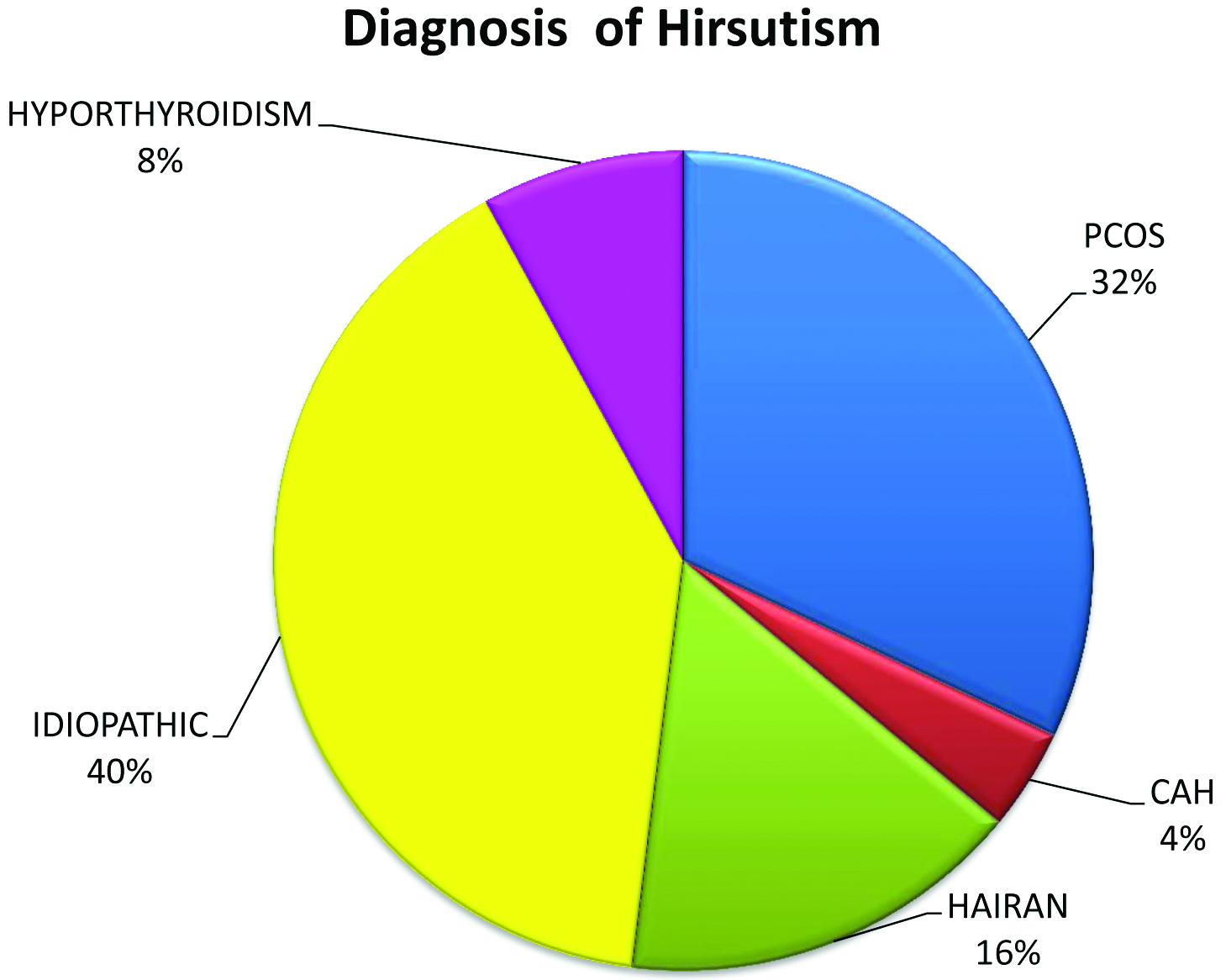
The mean age of the patients enrolled in the study was 29.42±10.83 years. The mean age of onset of symptoms was 21±4.2 years. The mean duration of symptoms was 3.75 (1-28) years. There were twenty six (52%) unmarried patients and twenty four (48%) married patients in the studied population. The causes of hirsutism (mF-G score >eight) were IH (40%), PCOS (32%), HAIR-AN (16%), hypothyroidism (8%), and late onset CAH (4%) [Table/Fig-2].
Aetiology of hirsutism.
*PCOS: Polycystic ovarian syndrome; IH: Idiopathic hirsutism; CAH: Congenital adrenal hyperplasia; HAIRAN: Hyperandrogenic insulin resistant acanthosis nigricans syndrome
There was history of regular menstrual cycle in thirty three patients (66%) and irregular menstruation in seventeen patients (34%). Of the patients with irregular menstruation, four patients (8% of total) had amenorrhea and thirteen (26% of total) had oligomenorrhea. PCOS was diagnosed in 88.2% of the patients with irregular menstrual cycle, of which two patients had amenorrhea and thirteen patients had oligomenorrhea. Significant association was noted between menstrual irregularity and PCOS (p-value <0.001, highly significant). Eleven patients (22%) of total sample had positive history of hirsutism in the first degree relative. Of the eleven patients of positive family history, five patients (10%) were diagnosed with PCOS, three (6%) had IH, two (4%) patient were diagnosed with late-onset CAH and one (2%) was hypothyroid. Thirty out of fifty patients were overweight (BMI≥25); twenty four had mild hirsutism, five had moderate, one had severe. The mean BMI for PCOS patients was 24.35±5.62 kg/m2, 27.00±6.75 kg/m2 in IH, 20.6±5.93 kg/m2 in CAH, 28.16±4.96 in HAIR-AN and 27.85±3.34 in hypothyroidism but comparison of mean weight amongst the different groups with one-way ANOVA revealed no statistical difference (p-value=0.3156). WC (>80 cm) was found in thirty two (64%) out of fifty patients but statistical association was seen in HAIR-AN patients only. Acne was present in nine out of sixteen patients of PCOS which was not statistically significant (p-value=0.24). Clitoromegaly was present in two (4%) patients both of which belonged to CAH. [Table/Fig-3] depicts above observations of patients.
Observations in study patients.
| Characteristic | Prevalence |
|---|
| Mean age of onset (years) | 21 (8-45) |
| Mean duration of symptoms (years) | 3.75 (1-28) |
| Mean age of presentation (years) | 29.42±10.83 (15-45) |
| Marital status (%) | 48 |
| Family history (%)• PCOS (%)• IH (%)• CAH (%)• Hypothyroidism (%) | 2210642 |
| Acne• Total (%)• Acne in PCOS (%) | 4056 |
| Regular menstrual cycle (%)Menstrual irregularitiesTotal (%)• Amenorrhea (%)• Oligomenorrhea (%)• Menstrual irregularity in PCOS (%) | 663482688.2 |
| Acanthosis nigricans | 20 |
| Overweight (BMI>25) (%) | 60 |
| Androgenetic alopecia (%) | 26 |
| Deepening of voice (%) | 4 |
| Clitoromegaly (%) | 4 |
| Waist circumference (>80 cm) (%) | 64 |
*BMI: Body mass index
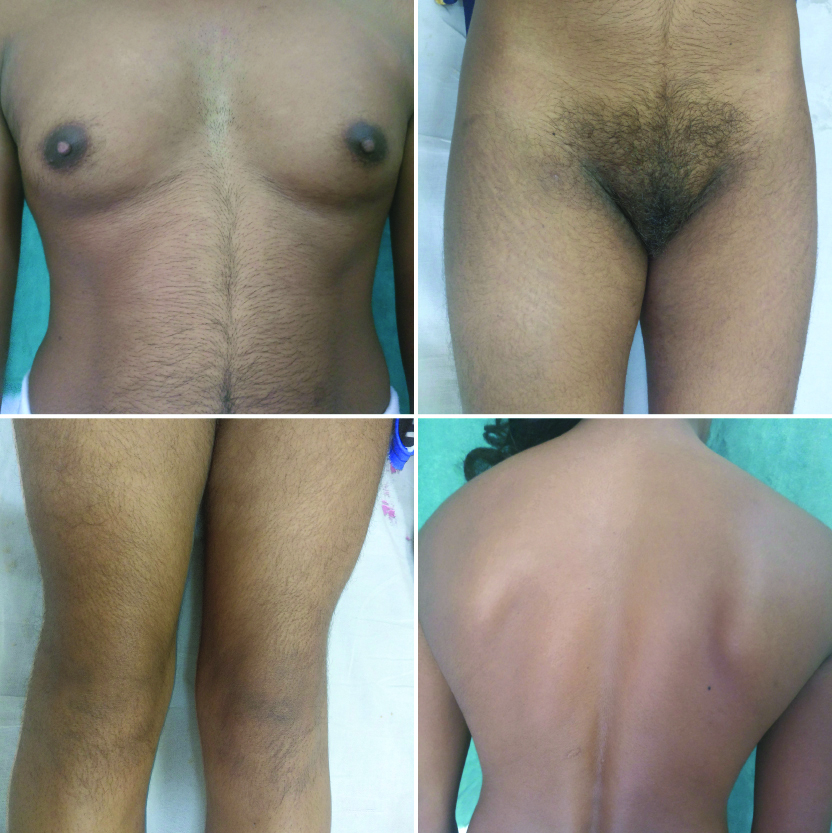
Forty one (82%) patients had mild hirsutism, seven (14%) patients had moderate hirsutism and two (4%) patients had severe hirsutism. Severe mF G score was observed in both patients of CAH [Table/Fig-4]. There was no significant difference in the mean values of mF-G score for PCOS and IH groups by a two-sample t-test for comparison, however the mean values of mF-G score were significantly higher for CAH than PCOS (p-value<0.001) [Table/Fig-5].
Late onset congenital adrenal hyperplasia with severe mF-G score, hypoplastic breasts and muscular habitus.
Mean values of waist circumference, modified Ferriman-Gallwey score and hormone profile in different aetiologies of hirsutism.
| Parameter | PCOS | IH | CAH | HAIR AN | HYPOTHYROIDISM | p-value |
|---|
| Waist circumference (cm) | 33.62±3.73 | 34.15±3.31 | 25±1.41 | 36.87±3.18 | 35.75±2.62 | 0.0014 |
| Body mass index (kg/m2) | 24.35±5.62 | 27.00±6.75 | 20.6±5.93 | 28.16±4.96 | 27.85±3.34 | 0.3156 |
| mF-G score | 12.93±3.15 | 11.95±2.89 | 30±5.65 | 12.25±4.30 | 15.75±2.87 | <0.001 |
| Serum free testosterone (Median) | 22.61±32.29 (0.9) | 22.14±36.9 (0.72) | 3.13±1.02 (3.13) | 5.14±10.53 (0.91) | 17.53±32.32 (1.78) | 0.6731 |
| Serum DHEAS | 2236.4±966.5 | 1933.1±622.66 | 2500±1838.5 | 2560.25±1067.5 | 1925±531.5 | 0.6620 |
| Serum prolactin | 13.23±7.39 | 12.28±6.31 | 12.16±8.09 | 11.62±5.72 | 14.29±10.15 | 0.9921 |
| Serum fasting insulin | 10.06±6.23 | 11.05±7.06 | 3.51±1.25 | 14.47±2.66 | 10.79±2.89 | 0.0302 |
| Serum 17-OH progesterone | 0.88±0.93 | 1.05±0.35 | 16±2.82 | 0.94±29 | 0.99±0.14 | <0.001 |
| Serum FSH | 6.28±3.95 | 4.25±1.72 | 6.31±2.53 | 2.97±1.18 | 3.75±2.48 | 0.6534 |
| Serum LH | 14.27±10.23 | 5.14±2.93 | 20.15±13.64 | 3.6±1.78 | 2.61±0.95 | <0.001 |
| LH: FSH ratio | 2.16±0.76 | 1.16±0.23 | 3.96±3.76 | 1.17±0.16 | 0.88±0.39 | <0.001 |
| HOMA-IR | 2.02±1.33 | 2.32±1.96 | 0.72±0.45 | 2.89±0.57 | 2.45±0.91 | 0.3564 |
*mF-G score: Modified Ferriman-Gallwey score; DHEAS: Dehydroepiandrosterone sulphate; FSH: Follicle stimulating hormone; LH: Leutinizing hormone; HOMA-IR: Homeostatic model assessment for insulin resistance; #Significant <0.05; Highly significant <0.001
[Table/Fig-6] shows ultrasound findings in various patients. Out of total 16 patients of PCOS, USG changes consistent with polycystic ovarian morphology were observed in 13 (81.25%) of the patients.
Ultrasonographic findings in patients.
| Ultrasonographic findings | N (%) |
|---|
| Normal | 31 (62) |
| Polycystic ovaries | 13 (26) |
| Unilateral (unilocular simple) cysts | 6 (12) |
| Adrenal pathology | 2 (4) |
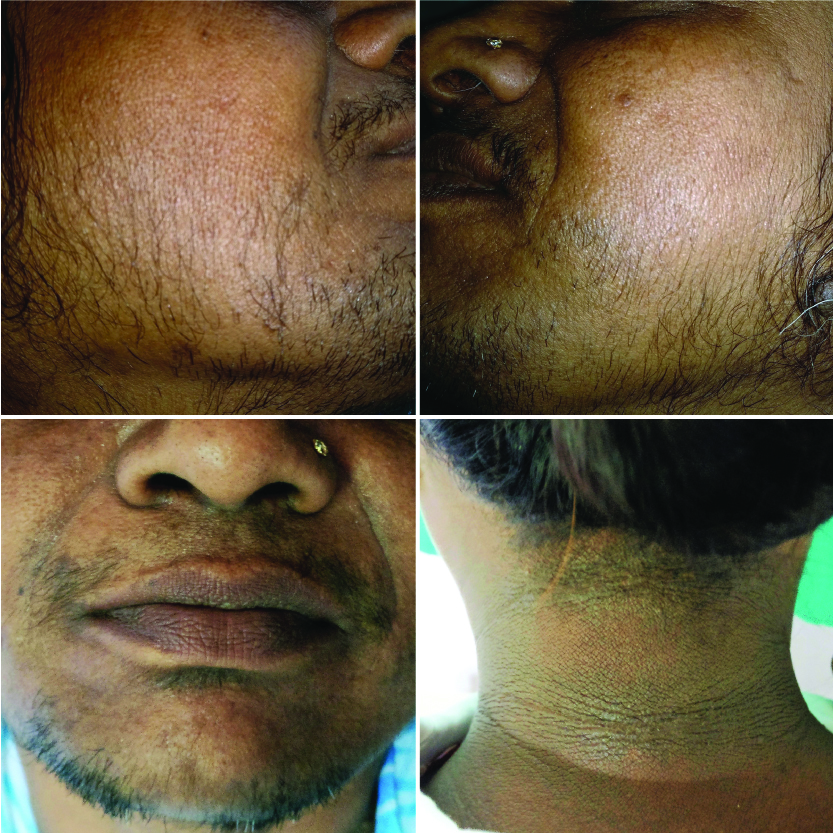
Serum 17-OH progesterone was highly significant in CAH as compared to other groups. Serum LH was highly significant in PCOS and CAH whereas LH:FSH ratio (>2) was significantly elevated in PCOS and CAH than IH. Circulating androgen levels (free testosterone) were raised in PCOS and IH women, while serum DHEAS were raised in HAIR-AN, CAH and PCOS, but there was no statistically significant difference between the groups. WC and serum fasting insulin were statistically significant in HAIR-AN group as compared to other groups. HOMA-IR was significantly raised in HAIR-AN group as compared to CAH but there was no statistically significant difference between other groups (PCOS, IH and hypothyroidism). [Table/Fig-7] showing clinical photograph of HAIR-AN patients. [Table/Fig-5] depicts mean values of WC, BMI, mF-G score and hormone profile in different aetiologies of hirsutism. Hypothyroidism was seen only in four patients (8%) in whom other hormonal profile was within normal limit. Serum prolactin level was raised only in one patient (2%) who had PCOS. Serum lipid profile was deranged in three patients (6%), one each of IH, PCOS and HAIR-AN syndrome. In all of these three patients, only hypertriglyceridemia was noted [Table/Fig-8].
HAIR-AN syndrome: Hirsutism patient with acanthosis nigricans.
| Characteristic | Prevalence (%) |
|---|
| ↓ Thyroid levels (%) | 8 |
| ← Serum prolactin level (%) | 2 |
| Deranged lipid profile-Hypertriglyceridemia (%)PCOSIHHAIR-AN | 6222 |
Discussion
Hirsutism is a common cosmetically disfiguring condition which runs a benign course. But in some cases, it can serve as a presenting feature for underlying hormonal and other systemic conditions which needs proper aetiological diagnosis and appropriate treatment. Hirsutism affects 5-10% of women of reproductive age [8]. The severity of hirsutism in different areas of the body depends on the rate of androgen excess, or increased sensitivity of hair follicles to normal androgen levels in the serum [4].
The pathogenesis of PCOS is multifactorial. It is associated with the abnormalities in steroid metabolism and control of androgen production. Ovarian theca cells produce androgens under the influence of LH from the pituitary gland and cytochrome P-450c17 (an enzyme with 17α-hydroxylase and 17,20-lyase-activity) induces the production of androstenedione [5]. This is then converted into testosterone by 17β-hydroxysteroid dehydrogenase or into estrone by aromatase. FSH from the pituitary gland regulates aromatase enzyme activity in granulosa cells and thereby the production of estrogens. Increased LH levels in PCOS patients as compared to the FSH, result in increased androgen production (testosterone) in the ovaries via stimulation of 17β-hydroxysteroid dehydrogenase and inhibition of aromatase [5].
Hirsutism occurs as a result of abnormally high androgen levels or by the presence of hair follicles which are more sensitive to normal androgen levels. Biologically active free testosterone is responsible for hair growth which is regulated by Sex Hormone-Binding Globulin (SHBG). The causes of androgenic hirsutism can be primary or secondary. Primary causes include excess endogenous androgen production of adrenal or ovarian origin; Ovarian hyperandrogenism can be due to PCOS and virilizing ovarian neoplasia (Luteoma of pregnancy, arrhenoblastomas, leydig cell tumours, hilar cell tumours, thecal cell tumours, etc.), adrenal hyperandrogenism can be due to congenital adrenal hyperplasia and adrenal tumours. Secondary causes include drugs like testosterone, dehydroepiandrosterone sulfate, danazol, corticotropin, high-dose corticosteroids, metyrapone, phenothiazine derivatives, anabolic steroids, androgenic progestin, and acetazolamide. Other rare causes include hyperprolactinemia, severe insulin resistance syndrome, hypothyroidism, cushing syndrome and acromegaly [5]. PCOS accounts for 75-80% cases of hyperandrogenism and clinically the most common sign of hyperandrogenism in PCOS is hirsutism. The prevalence of hirsutism in PCOS is reported to be between 17% and 83% [12].
IH (40%) was most common cause of hirsutism in our study which correlated well with Sharma D et al., and Ansarin H et al., and Carmina E, [3,7,13]. In current study, forty one (82%) patients had mild hirsutism (18 were of IH), seven (14%) patients had moderate hirsutism (2 were of IH) and two (4%) patients had severe hirsutism (both of which were of CAH). These observations were similar to Sharma D et al., (mild in 80% and moderate in 20%) and Ansarin H et al., (mild hirsutism in 65%, moderate in 32.5%, and severe in 2.5%) [3,7]. The maximum mF-G score was seen in chin followed by upper lip, chest and lower abdomen which was consistent with the finding of Sharma D et al., and Malik LM et al., who reported involvement of similar sites more commonly [3,14].
Mean age of the patients in our study was 29.42 which corresponded well with Sharma NL et al., and Zargar AH et al., where it was 25.8 and 26.1 respectively [4,6]. Family history in first degree relative was present in 22% patients which collaborated well with Zargar AH et al., where it was 25.4% [6]. Signs of virilisation were more common in PCOS patients in our study. Menstrual irregularities were present in 34% patients which correlated well with Chhabra S et al., Sharma D et al., Sharma NL et al., and Ansarin H et al., [1,3,4,7]. The observations of acne (40%), female pattern hair loss (26%) and obesity (38%) were parallel to Chhabra S et al., (55%, 27.5% and 37.5% respectively) [1]. AN was present in 20% patients which was consistent with findings of Sharma D et al. (26.3%) [3]. [Table/Fig-9] shows comparison of various characteristics between different studies [1,3,4,6,7].
Comparison between various studies.
| Characteristics | Present study | Chhabra S et al., [1] | Sharma D et al., [3] | Sharma NL et al., [4] | Zargar AH et al., [6] | Ansarin H et al., [7] |
|---|
| Mean age of the patients (years) | 29.42±10.83 | 24.18±5.61 | 23.8±6.66 | 25.8±8.30 | 26.1±9.5 | 20.9 |
| Most common cause | Idiopathic | PCOS | Idiopathic | NA* | Idiopathic | PCOS |
| Marital history present (%) | 48 | 17.7 | 20 | NA* | NA* | 26.8 |
| Family history (%) | 22 | 42.5 | 8 | 18 | 25.4 | 56.2 |
| Acne (%) | 40 | 55 | 60 | 64 | 8 | 70 |
| Menstrual irregularities (%) | 34 | 40 | 28 | 36 | 48 | 38 |
| Female pattern hair loss (%) | 26 | 27.5 | 6 | 16 | NA* | 21.3 |
| Acanthosis nigricans (%) | 20 | 37.5 | 26.32 | 6 | NA* | 4.9 |
| Obesity (BMI >30) (%) | 38 | 37.5 | 20 | NA* | 24.8 | 6.5 |
| WC (>80 cm) (%) | 64 | NA* | NA* | NA* | NA* | NA* |
*NA: Not available, WC: Waist circumference
The modified NCEP ATP III criteria have been formulated for diagnosis of metabolic syndrome [9,15]. According to these criteria, the presence of any three of the following five factors confirms the diagnosis of Metabolic Syndrome: abdominal obesity which is defined as WC >90 cm for men and >80 cm for women (for Asian origin), hypertriglyceridemia (triglycerides ≥150 mg/dL or 1.7 mmol/L); low HDL cholesterol (HDL cholesterol ≤40 mg/dL or 1.03 mmol/L for men and ≤50 mg/dL or 1.29 mmol/L for women); elevated blood pressure (systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg or on antihypertensive drugs); impaired glucose tolerance (fasting plasma glucose ≥100 mg/dL or 5.6 mmol/L) [15]. Using this criteria, WC >80 cm was observed in 64% of hirsutism patients, though statistical significance was noted in HAIR-AN syndrome patients. Hence, WC which forms a part of metabolic syndrome should be calculated in all hirsutism patients to detect metabolic syndrome at earliest and prevent its further complications.
Among the 16 patients of PCOS, Ultrasonographic (USG) changes were found in 13 (81.25%) patients. Sharma D et al., Ansarin H et al., and Hassa H et al., had reported similar findings in their respective study population [3,7,16].
CAH is characterised by underlying deficiency of 21-hydroxylase that leads to overproduction of 17-OH progesterone and androstenedione and consequently causing signs and symptoms of hyperandrogenism. It can be diagnosed in the neonatal period, during early childhood or later in puberty, where it is known as ‘late onset CAH’. They present with hirsutism, irregular menstrual cycles, and/or amenorrhea [5]. We found two cases of late onset CAH; both were sisters and were diagnosed with increased levels of 17-OH progesterone and androstenedione. CT scan showed bilateral adrenal hyperplasia in both patients. CAH is a rare cause of hirsutism but should be kept in mind especially when patients present with severe hirsutism.
Hypothyroidism was identified as a cause of hirsutism in 8% of patients. Similar observations were found by Chhabra S et al., and Sharma D et al., [1,3]. Serum TSH was not found to be statistically significant in any of the patients but all patients should be investigated for thyroid as they require thyroxine supplementation along with hirsutism management.
HAIR-AN syndrome also forms an important cause of hirsutism. In our study, we found 16% of patients of HAIR-AN syndrome. There are very few studies regarding the incidence of HAIR-AN syndrome in hirsutism patients. These patients develop hirsutism due to increased androgen production in the ovaries that is caused by Insulin like Growth Factor-1 (IGF-1) receptors of the ovarian theca cells [5]. Insulin also lowers SHBG serum levels leading to an increase in free testosterone in the peripheral blood [17]. Insulin has direct and indirect influence on hyperandrogenism in PCOS, stimulating the synthesis of steroid hormones in granulosa and theca cells. Stimulation of theca cells by insulin means an additional trigger for androgen production. Thus, due to hyperinsulinemia, PCOS patients have increased levels of free testosterone, while the total testosterone may be normal or only slightly increased [8]. This denotes the importance of measuring free testosterone levels rather than total testosterone levels. Hence, in women presenting with AN and hyperandrogenism, it is important to rule out HAIR-AN syndrome for appropriate management.
Limitation
Small sample size is a limitation of this study. In future, it is recommended that studies of hormonal profile of hirsutism with WC, BMI, serum fasting insulin, HOMA-IR, serum thyroid profile and metabolic syndrome criteria are taken up to evaluate its association with HAIR-AN syndrome, hypothyroidism and metabolic syndrome.
Conclusion
Hirsutism though a benign condition should be thoroughly evaluated. IH was most common aetiology of hirsutism in this study. WC and serum fasting insulin were statistically significant in HAIR-AN patients. HOMA-IR, though was significantly raised in HAIR-AN group as compared to CAH but not between other groups, highlights the importance of ruling out insulin resistance in patients presenting with hirsutism and AN as they require additional management in the form of metformin and lifestyle modifications. Hypothyroidism, though a rare cause should be suspected in patients with normal androgen profile, justifying the importance of serum thyroid profile in such cases. So it is suggested that investigative profile of hirsutism should also include WC, BMI, serum fasting insulin, HOMA-IR and serum thyroid profile routinely for precise targeted management.
*BMI: Body mass index
*mF-G score: Modified Ferriman-Gallwey score; DHEAS: Dehydroepiandrosterone sulphate; FSH: Follicle stimulating hormone; LH: Leutinizing hormone; HOMA-IR: Homeostatic model assessment for insulin resistance; #Significant <0.05; Highly significant <0.001
*NA: Not available, WC: Waist circumference