Patients with hip fractures generally belong to advanced age and often suffer from significant co-morbidities [1]. Hip fractures are painful and appropriate pain relief is imperative since inadequate pain relief can result in adverse outcomes like increased incidence of adverse cardiovascular events and delirium [2].
However, conventional pain treatment modalities (systemic opioids, non-steroidal anti-inflammatory drugs) have undesirable adverse-effects, many of those being particularly deleterious in elderly patients. [2-4]. This makes the role of multimodal analgesia all the more relevant for patients suffering from hip fractures. Ultrasound guided Fascia Iliaca Compartment Block (FICB) has been found to be effective, safe and reproducible technique for management of post-operative pain following hip surgeries [5]. The effect of single injection of FICB is, however limited by the duration of effect of block. Dexmedetomidine, an α-2 agonist, has been shown to prolong the duration of peripheral nerve blocks without any significant adverse effects [6,7]. Similarly, intravenous (IV) infusion of dexmedetomidine has successfully been used along with FICB [8].
There is still dearth of literature on the efficacy of dexmedetomidine for FICB, as only a few studies have evaluated this effect [9-11]. The present study was, therefore designed to study the effect of addition of dexmedetomidine to local anaesthetic agent ropivacaine in FICB for patients undergoing hip surgeries under subarachnoid block. The primary aim of the study was to evaluate whether addition of dexmedetomidine to Ropivacaine for Fascia iliaca block results in prolongation of effects of block with secondary aim of looking for any adverse effects.
Materials and Methods
The study was conducted in a prospective randomised double blinded manner in a tertiary level hospital after approval from hospital ethics committee (DMCH/R&D/2014/44). Study was conducted from 15th January 2013 to 30th June 2015. The sample size was calculated by post hoc power analysis conducted using the software package, G power [12]. Based on a previous study by El-Rahmawy GF et al., using reduced analgesic requirement parameter, with an alpha level of p<0.05 and beta of 0.20, assuming 10% error due to chance, a sample size of 26 patients in each of two groups was required for a power of 80% [10]. However, 30 patients were included in each group to account for any possible exclusions or drop outs.
Sixty adult American Society of Anaesthesiologists (ASA) class I-III patients of either sex scheduled for elective hemi-arthroplasty under Subarachnoid Block (SAB) were included in the study after written informed consent. Patients with refusal or failure of SAB, uncooperative patients or patients with communication difficulties, peripheral neuropathies or myopathies on the operative limb, severe liver and kidney dysfunction, history of allergy to any drug being used in study, vascular disease of limbs and history of chronic pain, were excluded from the study.
All patients underwent a thorough pre-anaesthetic check up comprising of history, general physical and systemic examination a day prior to surgery. As an institutional protocol, all patients underwent investigations which included complete haemogram, bleeding time, clotting time, prothrombin time index, urine routine examination, Fasting blood sugar, Electrocardiography, X-ray chest and other investigations if indicated.
During the pre anaesthesia interview, patients were introduced to and instructed about Visual Analogue Scale (VAS) as a tool for measuring post-operative pain. VAS scale which consists of a 10 cm line, having markings at 1 cm each, was shown and the patients were explained that they have to bring the slider on the scale on to the point that they feel represents their state of pain with ‘0’ mark corresponding to no pain and ‘10’ mark representing worst imaginable pain. Patients were also educated on the post-operative pain relief plan and informed about their right to request rescue analgesic if needed at any time during the study period.
All the patients were kept nil orally for at least 6-8 hours prior to surgery as per ASA fasting guidelines. Premedication in the form of Tab Alprazolam 0.25 mg orally at night before surgery and 0.25 mg at 6 AM on the day of surgery with a sip of water was given to all the patients. On the day of surgery, after shifting the patient to operation theatre, monitoring of Heart Rate (HR), Non-Invasive Blood Pressure (NIBP), Respiratory Rate (RR), peripheral oxygen saturation (SpO2) and Electrocardiogram (ECG) was done. After achieving an intravenous access, patients were preloaded with 0.9% normal saline 10 mL/kg body weight over 20 minutes. Then, under all aseptic precautions, spinal anaesthesia (2.6 mL of plain 0.5% heavy bupivacaine) was given in lateral position. Patients were monitored continuously for HR, RR, SpO2, Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP) and Mean Arterial Pressure (MAP). These vital parameters were recorded every 5 minutes by the anaesthetist managing the case. Surgery was allowed to proceed after achieving successful spinal block, defined as absence of pin prick sensation at or above T10 dermatomal level. Patients were randomly allocated to one of the two groups of 30 patients using computer generated random numbers, kept in sealed envelopes which were opened at the end of study. The drug to be administered was prepared by a blinded anaesthetist, not involved in the administration of the FICB or observations. After the completion of procedure, patients were administered FICB under ultrasound guidance by a single trained anaesthetist.
Group A patients were administered FICB by injecting 3 mg/kg of ropivacaine whereas, Group B Patients received FICB using 3 mg/kg of ropivacaine along with 1 μg/kg Dexmedetomidine as an adjuvant [11]. The respective drug combinations were made to a fixed volume of 40 mL of normal saline by a person not involved in the study and marked with a coded label to maintain double blind nature of the study.
With patient lying supine and leg extended, area from umbilicus to mid thigh was cleaned with 10% povidone iodine and draped with sterile sheets. A line drawn between the anterior-superior iliac supine and the pubic tubercle was divided into 3 equal parts and labelled with a marking pen. Using linear ultrasound transducer, iliacus muscle, iliacus fascia, femoral nerve, femoral artery and femoral vein were identified. Ultrasound transducer was then moved laterally along the fascia iliaca to its junction at the Sartorius muscle.
The target injection site was determined at the junction of the lateral and middle thirds of the line connecting the anterior-superior iliac spine and the pubic tubercle at the intersection of the iliacus and Sartorius muscles. A 21 gauze, 10 cm needle (stimuplex) was inserted in-plane from lateral to medial at level of the femoral crease to cross the fascia iliaca at the junction of the Sartorius and iliacus muscles. An injection was considered successful when the spread of Local Anaesthesia (LA) reached the femoral nerve medially and at least three cm laterally from the point of injection beneath the fascia iliaca towards the anterior-superior iliac spine. If the medial-lateral spread of LA was not accomplished with a single injection, additional injections were made. Time of administration of FICB was labelled as 0 minutes and patients shifted to Post Anaesthesia Care Unit (PACU). The observations in PACU were made by an independent observer, blinded to the group allocation of the patient. On arrival to PACU, vital parameters and VAS were monitored at 30 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, 12 hours, 16 hours, 20 hours and 24 hours.
Patients were administered rescue analgesia in the form of Inj Tramadol 50 mg IV, whenever they complained of pain or requested analgesia or reported VAS ≥4. Injection tramadol IV could be repeated after 15 minutes if patient still complained of pain, subject to a maximum of 100 mg in 4 hours or 300 mg in 24 hours. If these dose thresholds were breached and patients still complained of pain, such patients were excluded and treated according to discretion of the anaesthetist managing the patient. Time of first administration of rescue analgesic and total analgesic consumption in 24 hours was noted. Patients were also observed for any adverse effects like nausea and vomiting, skin rash, tachycardia (defined as HR > 100/min), bradycardia (defined as HR < 50/min), Hypotension (SBP < 90 mmHg), Hypoxemia (SpO2 <90%), sedation, or any other side effect, if any, during 24 hours post-operative period. Sedation was assessed using Ramsay sedation scale [13].
Statistical Analysis
Data was described in terms of mean ±standard deviation (±SD). Comparison of quantitative variables between the study groups was done using Student’s t-test. Chi square (χ2) test was performed and Fisher’s-exact test was used when the expected frequency was less than 5. A probability value (p-value) less than 0.05 was considered statistically significant. All statistical calculations were done using SPSS (Statistical Package for the Social Science) SPSS 21 version statistical program for Microsoft Windows.
Results
All patients entering the study were analysed and none of the patients were excluded [Table/Fig-1]. Both the groups were demographically similar with respect to mean age, weight, height and sex and ASA class distribution [Table/Fig-2].
Flow chart of patient’s recruitment.
| Group A (n=30) | Group B (n=30) | p-value |
|---|
| Mean age (years) | 57.25±14.345 | 65.15±18.328 | 0.137 |
| Sex distribution Male: Female | 9:11 | 9:11 | 1.000 |
| Mean weight (kg) | 69.30±15.124 | 64.00±13.338 | 0.247 |
| Mean height (cm) | 165.00±8.944 | 162.90±6.719 | 0.406 |
| BMI | 25.15±3.686 | 23.85±4.477 | 0.322 |
| ASA grade I:II:III | 5:13:2 | 3:13:4 | 0.558 |
| Duration of surgery (min) | 104.17±24.38 | 116.38±19.59 | 0.550 |
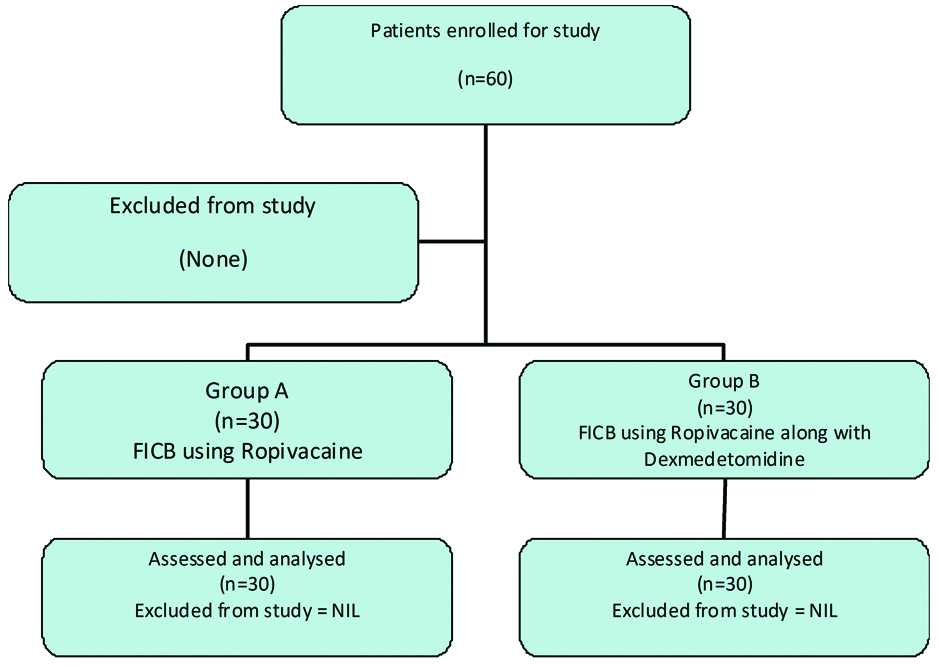
The mean baseline heart rates were comparable between group A and group B (p-value=0.185). Later on, heart rate was higher in group A as compared to group B at most time intervals, though the difference was statistically insignificant. Mean heart rate however, was significantly higher in group A at 30 minute, 1 hour, 2 hour and 4 hour after administration of FICB [Table/Fig-3].
Mean heart rate after FICB.
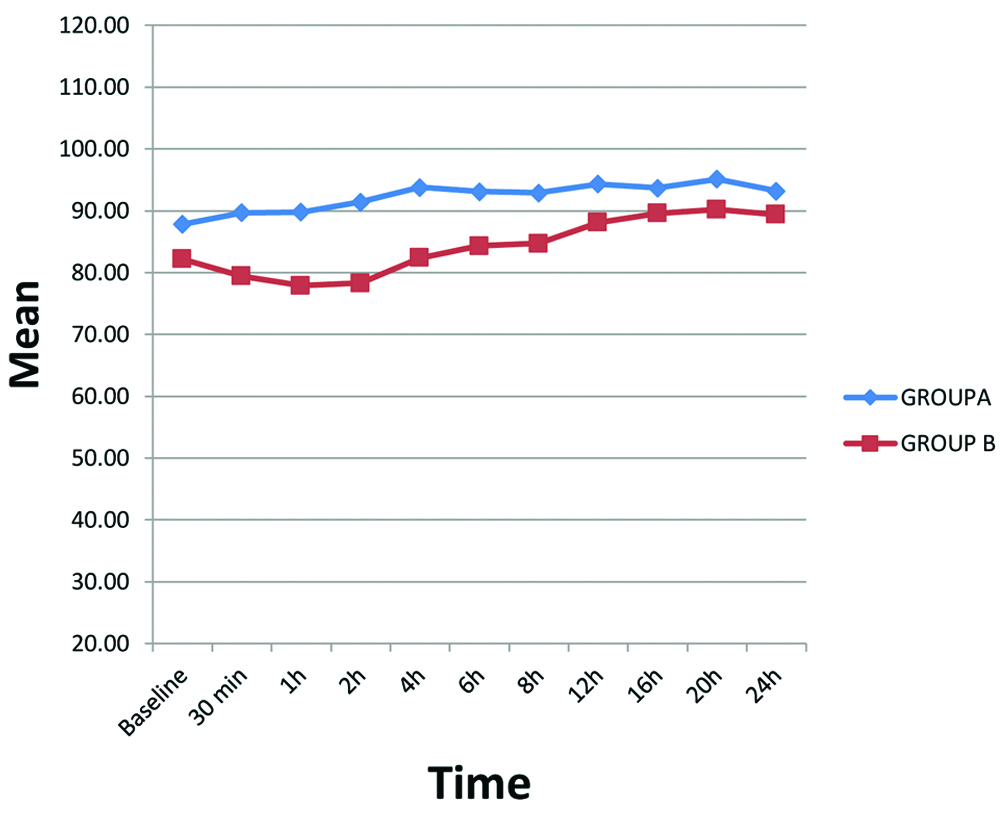
The mean baseline MAP in group A and group B was statistically similar at 100.60±10.77 and 95.80±16.59 mmHg respectively (p=0.285). With time, MAP was higher in group A as compared to group B, though the difference was statistically insignificant. However, MAP was statistically significantly greater in group A at 6th hour and 16th hour post FICB [Table/Fig-4].
Mean blood pressure after FICB.
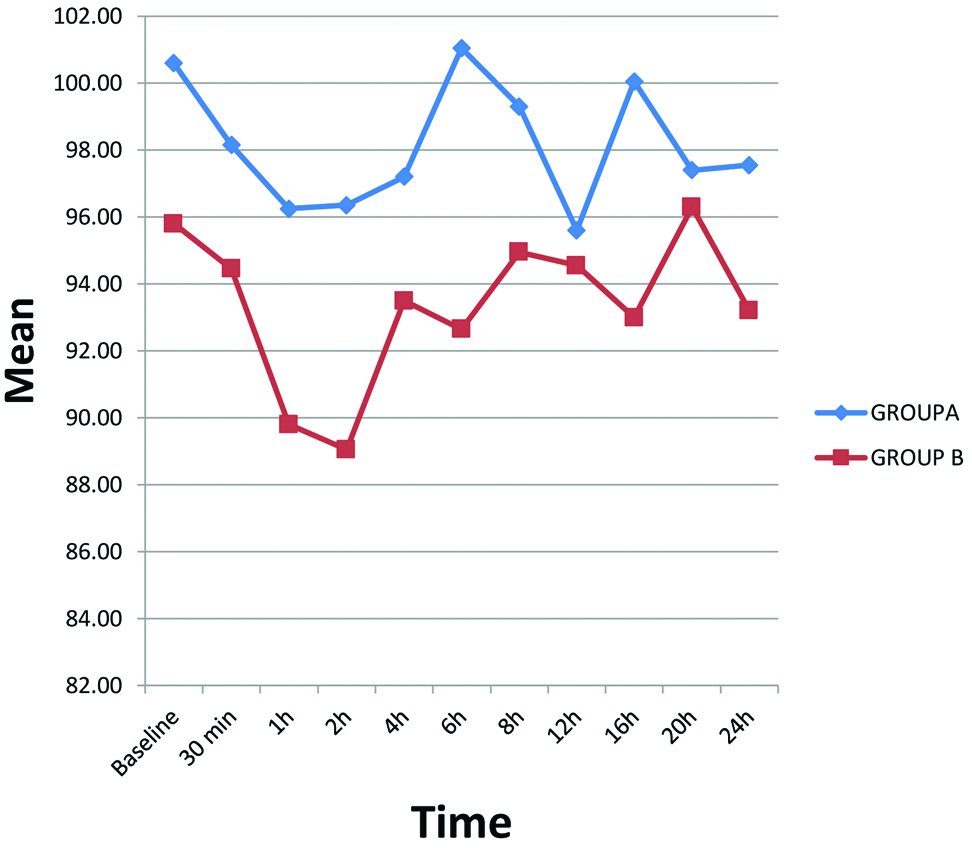
Mean baseline VAS was zero in both the groups and mean VAS remained higher in group A at all time intervals though the difference was statistically not significant (p=0.770) [Table/Fig-5].
Mean VAS among the two groups.
After FICB administration, mean duration of time when patient complained of pain in group A was 275.29±226.31 minutes while in group B, it was 465.8±325.56 minutes and the difference was statistically significant (p=0.036). Mean VAS at that time was 5.59±1.06 in group A while in group B, it was 5.71±0.77. The difference in mean VAS when patient complained of pain which was statistically not significant (p=0.716) [Table/Fig-6].
Mean rescue analgesic consumtion.
| Group A (n=30) | Group B (n=30) | p-value |
|---|
| Median number of rescue analgesics given along with Interquarantile Range (IQR) | 3 (2-3.5) | 2 (1-3) | 0.015 |
| Mean total analgesic consumption in 24 hours | 207.24±45.17 | 145.56±48.31 | 0.015 |
| Mean time of administration of first analgesic (min) | 275.29±226.31 | 465.8±325.56 | 0.036 |
| VAS at time of first rescue analgesic | 5.59±1.064 | 5.71±0.772 | 0.716 |
Similarly, patients in group A required rescue analgesia more frequently in the initial 24 hours as compared to group B and this difference was statistically significant [Table/Fig-6]. The median number of times along with Interquartile range when rescue analgesia was required in group A was 3 (IQR 2-3.5) whereas in group B, it was 2 (IQR 1-3). (p=0.015) [Table/Fig-6]. Mean total analgesic consumption in 24 hours was also significantly less in group B at 145.56 ±48.31 mg as compared to 207.24±45.17 mg in group A (p=0.015) [Table/Fig-6].
None of the patients in either of the groups had adverse effects such as hypotension, skin rash, tachycardia, bradycardia or hypoxemia. Three patients in group B had sedation score of 4, while all patients in Group A had sedation scores of 3 or less. However, the difference in sedation scores was statistically not significant. (p=0.072).
Discussion
Fascia iliaca block is a safe, reproducible and effective technique for pain management [14]. The benefits of adequate analgesia employing FICB as one of the modalities includes improved cognition, better patient satisfaction and reduction of complications [11]. FICB blocks have been found to be more effective than femoral nerve blocks in alleviating post operative pain after hip surgeries [15,16]. Ropivacaine was chosen as local anaesthetic agent as it has significantly less neurotoxic and cardiotoxic potential compared to bupivacaine and is also longer acting compared to lignocaine. Its vasoconstrictive property makes it less toxic by preventing sharp increase in plasma concentration of the local anaesthetic [17]. Dexmedetomidine, used as an adjunct to ropivacaine for FICB in our study is a highly selective central alpha-2 agonist and has sedative, anxiolytic and analgesic properties [18,19]. IV infusion of dexmedetomidine has been found to reduce pain scores when used in conjunction with FICB [8]. Various studies conducted in the past have reported that addition of dexmedetomidine as an adjuvant to ropivacaine for peripheral nerve block prolonged the duration of analgesia and time to first rescue analgesia with improved quality of block [6,20]. In our study, none of the patients were excluded and both the groups were statistically comparable with respect to age, weight and ASA grade. It was observed that patients in group B, received rescue analgesic after a mean of 465.8 minutes as compared to 275.29 minutes in group A. This implies that group B patients required analgesia for a mean 190.51 minutes later as compared to group A and this difference was statistically significant. Our results were similar to the findings of Lin YN et al., who also reported prolongation of the duration of sensory block by mean of 1 hour after addition of dexmedetomidine to ropivacaine for cervical plexus block [6]. In another study, by Fritsch G et al., the median duration of sensory block was prolonged by 4 hours in dexmedetomidine group when used in interscalene block [20].
Rescue analgesia was required less often in group B, thus indicating that dexmedetomidine reduces analgesic requirement. Similarly, mean total analgesic consumption in 24 hours was significantly less in group B (145.56±48.31 mg) than in group A patients (207.24±45.17 mg), thus implying that addition of dexmedetomidine to ropivacaine in FICB has opioid sparing effect. El-Rahmawy GF et al., also observed that time to administration of rescue was significantly later in the patients who received dexmedetomidine in local anaesthetic for FICB. In addition, dexmedetomidine group had significantly lower VAS scores and decreased rescue analgesic consumption [10].
Heart rate was higher in group A than in group B and the difference was statistically significant at 30 minute, 1st hour, 2nd hour, 4th hour post FICB. Similarly, MAP was lower in group B than group A and the difference was statistically significant at 6th hour and 16th hour post FICB. Our findings of greater haemodynamic stability in group B are similar to the finding by Lin YN et al., where MAP level and HR level were significantly lower in patients receiving ropivacaine and dexmedetomidine as compared to patients who received ropivacaine alone [6]. EL-Rahmawy GF et al., also reported lower HR and MAP in the dexmedetomidine group [10]. The reason for greater haemodynamic stability may be attributed to be due to better pain relief in Group B. The mean VAS was similar in both the groups and this could be explained by the fact that whenever, patients reported VAS ≥4, a dose of rescue analgesic was administered. The possible mechanism by which dexmedetomidine exerts its effect include its peripheral and central actions. Peripherally, α-2 agonists produce analgesia by reducing release of norepinephrine and causing α-2 receptor-independent inhibitory effects on nerve fibre action potentials. Centrally, α-2 agonists produce analgesia and sedation by inhibiting substance P release in the nociceptive pathway at the level of the dorsal root neuron and by activating α2 adrenoceptors in the locus coeruleus [21,22]. Therefore, addition of dexmedetomidine as an adjuvant to ropivacaine for post-operative pain relief in FICB block prolonged the duration of block and improved its quality. In group B, sedation was observed in three patients, which might have occurred from absorption of dexmedetomidine from the local site while there were no side effects seen in group where only ropivacaine was used for FICB. The efficacy of dexmedetomidine as an adjuvant for peripheral neuraxial blocks like brachial plexus block has been reported by various studies [6,7,20]. The results of present study suggest that the addition of dexmedetomidine in a dose of 1 mcg/kg to ropivacaine is effective in prolonging the effect of FICB in patients undergoing hemi-arthroplasty, without any significant adverse effects.
Limitation
The limitation of our study which is worth mentioning is that only a single dose of dexmedetomidine as an adjuvant for FICB was evaluated and the patients operated upon by different surgeons were included, thus introducing possibilities of differences in tissue handling and resulting pain. Further dose-response studies may be planned to find out an optimal dose for its use as an adjuvant in FICB.
Conclusion
The addition of dexmedetomidine to ropivacaine for FICB block significantly prolongs the effect of block and decreases total dose of rescue analgesic consumption in patients undergoing hemi-arthroplasty.