Multidrug-Resistant Organisms (MDROs) particularly staphylococci have emerged as important causes of Healthcare-associated infections (HAIs), and these infections are related with significant morbidity and mortality [1]. ESRD patients are at higher risk in acquiring infection and colonisation with methicillin-resistant staphylococci (MRS) because they are repeatedly exposed to the hospital environment and often receive prolonged courses of antibiotics, besides being immunocompromised. Haemodialysis unit provides an ideal setting for the cross-transmission of these pathogens and this might occur either through direct patient to patient contact or indirectly through the contaminated hands of hospital staff or environmental surfaces [1,2]. In haemodialysis patients, knowing the carrier state is important for predisposing to subsequent infections and also it can transfer the organisms among dialysis unit staff and community.
There are numerous reports which reveal that there is an estimated prevalence of colonisation of 30% with Staphylococcus aureus and 100% with CoNS which are both commensal and opportunistic pathogens [3,4]. Among CoNS, S. epidermidis, S. haemolyticus and S. hominis are major components of mucosal flora including the nasal microbiota and human skin. Rates of nasal colonisation with methicillin-resistant (MR) isolates are usually lower in the community and tend to increase in the hospital environment, and are much higher among CoNS than S. aureus [3-5]. This is one of the major reasons for CoNS being regarded as reservoirs of methicillin resistance determinant (mecA). Methicillin resistance, mediated by mecA gene encoding low affinity Penicillin-Binding Protein (PBP) 2a, has been observed in high proportion in CoNS isolates [6].
It is also reported that MR-CoNS are resistant to other antimicrobial agents compared to MRSA in addition to resistance to methicillin. There has been an indication that there may be a horizontal transfer of these genes among staphylococci, as many of these resistant genes in CoNS are located on MGEs and similar genes have been identified in S. aureus [6-11]. The acquisition of MDR determinants is a characteristic of MR-CoNS making them dangerous pathogens thereby complicating their successful treatment and control.
The accurate and rapid diagnosis of antibiotic resistance genes and associated SCCmec types in staphylococcal carriage is extremely important particularly among haemodialysis patients who are prone to infections. To date, only a few studies have been reported that examined the antimicrobial resistance patterns among individual CoNS species, making the prevalence of antimicrobial resistance among individual species difficult to ascertain [12]. Hence, the present study was aimed to detect the species distribution and its antibiotic resistant genes and to detect the diversity of SCCmec types among nasal carriage of MR-CoNS from ESRD patients and dialysis unit staff in Chennai, South India.
Materials and Methods
This cross-sectional prospective study was conducted over a period of two months (August-September 2013) at the nephrology unit of a tertiary care hospital, Chennai, Tamil Nadu, India. The study was approved by Institutional Human ethical committee (IEC No. PGIBMS/CO/Human Ethical/2011-12/546). Informed consent was obtained from all the subjects included in this study. Hospital records of patients including name, age, sex, duration of dialysis, associated risk factors and co-morbid conditions were documented.
(a) Sample Collection
A total of 145 nasal swabs were collected, of which 115 were from haemodialysis patients and 30 from dialysis unit staff. Using sterile Hi culture collection cotton swabs (HiMedia), the anterior nasal areas were swabbed and transported immediately to the laboratory for the identification of organisms.
(b) Isolation and Identification of Staphylococci
The collected nasal swabs were immediately processed by using 7.5% salt nutrient broth at 37°C for 2-4 hour for initial enrichment of staphylococci. Following enrichment, the tubes containing the swabs were vortexed for 15s and then sub-cultured onto blood agar, MacConkey agar (HiMedia) and incubated at 37°C for 24 hours; for selective isolation of staphylococci, mannitol salt agar (HiMedia) was employed. Based on the gram stain, colony morphology and mannitol fermentation, the isolates were confirmed to be staphylococci by the following standard laboratory tests {Catalase test, slide and tube coagulase tests and DNAse test (DNAse agar base, Hi Media)} [Table/Fig-1].
Isolation and identification of staphylococci.

(c) Screening of methicillin-resistance by phenotypic and genotypic methods
Methicillin resistance was screened by using cefoxitin (30 μg) disc diffusion method and Multiplex PCR (M-PCR) was performed for the simultaneous detection and differentiation of MRSA from MR-CoNS [13]. S. aureus ATCC 43300 and S. epidermidis 35984 were used as positive control.
(d) Speciation of MR-CoNS isolates
Speciation of MR-CoNS isolates was performed manually by using following biochemical tests namely; alkaline phosphatase test, haemolysis on blood agar, urease test, sugar fermentation (mannitol, sucrose, maltose, mannose, trehalose), polymixin B and novobiocin susceptibility [14].
Molecular identification was carried out for S. epidermidis and S. haemolyticus by species specific PCR to differentiate both the organisms [15]. The following primers were used SE1 (ATCAAAAAGTTGGCGAACCTTTTC) and SE2(CAAAAGAGCGTGGAGAAAAGTATCA); SH1 (GGTCGCTTAGTCGGAACAAT), SH2 (CACGAGCAATCTCATCACCT). The PCR cycle conditions were: denaturation for 3 min at 92°C, followed by 30 cycles of 92°C for 1 minute, 56°C for 1 minute and 72°C for 1 minute, and a final extension at 72°C for 5 minute. Amplified products were analysed by 2% agarose gel electrophoresis.
(e) Antimicrobial susceptibility testing (AST)
AST was done for the following antibiotics using Kirby-Bauer disc diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines 2012 [16]. The following antimicrobial agents were tested: amikacin (30 μg), ciprofloxacin (5 μg), clindamycin (2 μg), co-trimoxazole (1.25/23.75 μg), erythromycin (15 μg), fusidic acid (30 μg), Gentamicin (10 μg), linezolid (30μg), ofloxacin (5 μg), tetracycline (30 μg), vancomycin (30 μg). S. aureus ATCC 25923 and S. epidermidis ATCC 12228 were used as control strains. Minimum Inhibitory Concentration (MIC) of linezolid was done by agar dilution method and with HiCombTM MIC strips (HiMedia).
(f) Detection of inducible and constitutive clindamycin resistance
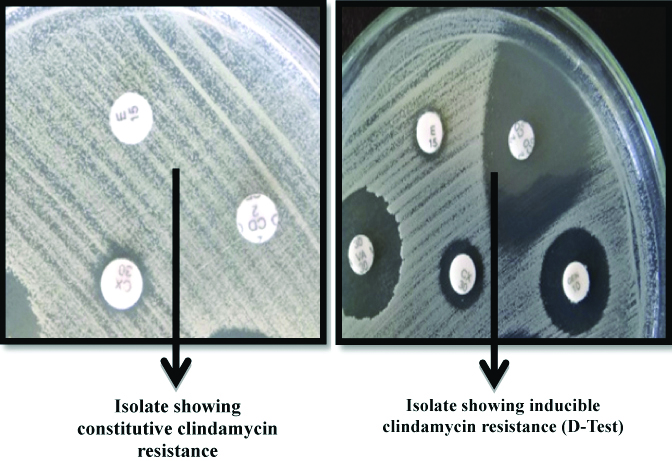
Inducible and constitutive clindamycin resistance was detected among all the erythromycin-resistant isolates by the double-disc test method with erythromycin (15 μg) disc and clindamycin (2 μg) disc [16]. The inducible phenotype was characterised by a positive D test, a flattening of the inhibition zone around the clindamycin disc near the erythromycin disc which indicated positive inducible macrolide-lincosamide-streptogramin B (iMLSB). The phenotype constitutive (cMLSB) was characterised by erythromycin and clindamycin resistance [Table/Fig-2]. Macrolide streptogramin phenotype (MSB) was characterised by clindamycin sensitivity and erythromycin resistance, with negative D test.
Detection of constitutive and inducible clindamycin resistance.
(g) PCR-based detection of antimicrobial resistance genes
Erythromycin, tetracycline and aminoglycoside resistant genes msrA, erm (A), erm (C), tet (K) tet (M) and aac (6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa, ant(6)-Ia, ant(4′)-Ia respectively were determined by M-PCR [17-19]. Fusidic acid resistant genes (fusB, fusC & fusD) were determined by the method of Castanheira M et al., [20]. Linezolid resistant cfr gene and point mutation in domain V of 23S rRNA gene were detected by using previously described method [Table/Fig-3] [21,22].
Primers and their sequences for various antibiotic resistance encoding genes, SCCmec types and SCCmec type IV subtypes used in this study [13,17-24].
| Target genes | Primer sequences | Product (bp) | Reference |
|---|
| ant (4’)-Ia | F: ATGGCTCTCTTGGTCGTCAGR: TAAGCACACGTTCCTGGCTG | 367 | |
| aph (3’)-IIIa | F: CGATGTGGATTGCGAAAACTR: CACCGAAATAACTAGAACCC | 175 |
| aac (6’)-Ieaph (2’’) | F: CATTATACAGAGCCTTGGGAR: AGGTTCTCGTTATTCCCGTA | 279 |
| erm (A) | F: AAGCGGTAAACCCCTCTGAR: TTCGCAAATCCCTTCTCAAC | 190 | Strommenger B et al., [18] |
| erm (C) | F: AATCGTCAATTCCTGCATGTR: TAATCGTGGAATACGGGTTTG | 299 |
| tetK | F: GTAGCGACAATAGGTAATAGTR: GTAGTGACAATAAACCTCCTA | 360 |
| tetM | F: AGTGGAGCGATTACAGAAR: CATATGTCCTGGCGTGTCTA | 158 |
| 23S rRNA | F: 5’-GCGGTCGCCTCCTAAAAGR: 5’-ATCCCGGTCCTCTCGTACTA | 390 | Meka VG et al., [22] |
| cfr | F: 5’-TGAAGTATAAAGCAGGTTGGGAGTCAR: 5’-ACCATATAATTGACCACAAGCAGC | 746 | Kehrenberg C et al., [21] |
| fusB | F: TCATATAGATGACGATATTGR: ACAATGAATGCTATCTCGAC | 496 | Castanheira M et al., [20] |
| fusC | F: GATATTGATATCTCGGACTTR: AGTTGACTTGATGAAGGTAT | 128 |
| fusD | R: TGCTTATAATTCGGTCAACGR: TGGTTACATAATGTGCTATC | 525 |
| msrA | F: GAAGCACTTGAGCGTTCTR: CCTTGTATCGTGTGATGT | 287 | Shittu AO et al., [17] |
| dfrA | F: CTCACGATAAACAAAGAGTCAR: CAATCATTGCTTCGTATAACG | 201 |
| mecA | F: TGCTATCCACCCTCAAACAGGR: AACGTTGTAACCACCCCAAGA | 286 | Nagarajan A et al., [13] |
| femA | F: AAAAAAGCACATAACAAGCGR: GATAAAGAAGAAACCAGCAG | 132 |
| SCCmec typing |
| ccrA2-B2 | F: ATTGCCTTGATAATAGCCYTCTR: TAAAGGCATCAATGCACAAACACT | 937 | Boye K et al., [23] |
| ccrC | F: CGTCTATTACAAGATGTTAAGGATAAT R: CCTTTATAGACTGGATTATTCAAAATAT | 518 |
| IS1272 | F: GCCACTCATAACATATGGAAR: 5’-CATCCGAGTGAAACCCAAA | 415 |
| IS431 | F: TATACCAAACCCGACAACTACR: CGGCTACAGTGATAACATCC | 359 |
| Subtyping of SCCmec type IV (IVa-IVg) |
| ccrB2 | F: CGAACGTAATAACATTGTCGR: TTGGCWATTTTACGATAGCC | 203 | Milheirico C et al., [24] |
| IVa | F: ATAAGAGATCGAACAGAAGCR: TGAAGAAATCATGCCTATCG | 278 |
| IVb & IVf | F: TTGCTCATTTCAGTCTTACCR: TTACTTCAGCTGCATTAAGC | 336 |
| IVc & IVE | F: CCATTGCAAATTTCTCTTCCR: CCATTGCAAATTTCTCTTCC | 483 |
| IVd | F: TCTCGACTGTTTGCAATAGGR: CAATCATCTAGTTGGATACG | 575 |
| IVg | F: TGATAGTCAAAGTATGGTGGR: GAATAATGCAAAGTGGAACG | 792 |
Nucleotide Sequence Accession Number
The nucleotide sequences of a cfr gene, 23S rRNA mutation, fusB, fusC and aac (6)-Ie-aph (2)-Ia gene were deposited in Gen Bank (NCBI) with the accession number KF744025, KF790758, KJ802933, KM411357 and KM411358 respectively.
(h) Detection of SCCmec types and SCCmec type IV subtypes
SCCmec typing (type I-V) was done by using M-PCR [23]. Positive control strains used in the determination of the SCCmec type were the MRSA strains COL-SCCmec type I, Mu50- SCCmec type II, SCCmec type III- ANS46, SCCmec type IV- MW2, SCCmec type V- WIS. SCCmec IV subtypes (IVa, IVb/IVF, IVc/IVE, IVd, IVg, IVh) were determined by M-PCR with primers described by Milheiriço C et al., [Table/Fig-3] [24].
Statistical Analysis
A simple standard deviation was carried out for age distribution analysis of ESRD patients and hospital personnel.
Results
The age distribution of patients who were undergoing haemodialysis and hospital personnel are listed [Table/Fig-4]. The associated risk factors and co-morbid conditions of the ESRD patients included in this study are mentioned in [Table/Fig-5]. A total of 135 isolates of staphylococci were obtained from 145 samples giving an isolation rate of 93%. Of these, 105/115 (91%) were from ESRD patients and 30/30 (100%) were from hospital personnel who were found to be colonisers of staphylococci. Ten samples were negative for any growth upon culture. 79 (58.5%) isolates were found to be methicillin resistant by cefoxitin disc diffusion method and M-PCR [Table/Fig-6].
Age-wise distribution of ESRD patients and hospital personnel.
| Details of patients and hospital personnel | Average age (mean±SD, years) |
|---|
| Haemodialysis patients (n=115) |
| Male (n=68) | 46.2±16 |
| Female (n=47) | 47±16.7 |
| Hospital personnel (n=30) |
| Male (n=10) | 37.2±7.11 |
| Female (n=20) | 30.2±10.8 |
SD: Standard deviation
Associated risk factors and co-morbid conditions of the ESRD patients.
| S. No. | Factors and conditions | No. of patients | Percentage |
|---|
| Associated risk factors |
| 1. | Hypertension | 56 | 49% |
| 2. | Diabetes mellitus | 32 | 28% |
| 3. | Smoking | 10 | 8.7% |
| 4. | Alcoholism | 6 | 5.2% |
| Co-morbid conditions |
| 5. | Graft rejection | 2 | 1.7% |
| 6. | Polycystic kidney disease | 2 | 1.7% |
| 7. | Hepatitis-C infection | 9 | 7.8% |
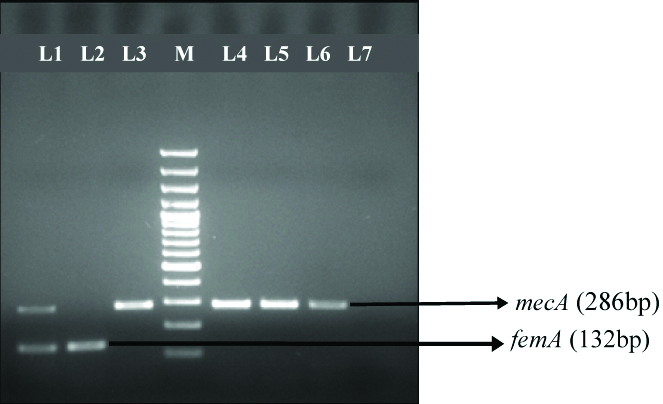
Multiplex PCR for detection of MR-CoNS and simultaneous differentiation from MRSA.
L1- S. aureus ATCC 43300; L2- S. aureus ATCC 25923, L3- S. epidermidis ATCC 35984; L4; L5; L6- MR- CoNS; L7- S. epidermidis 12228; M- 100 base pair ladder
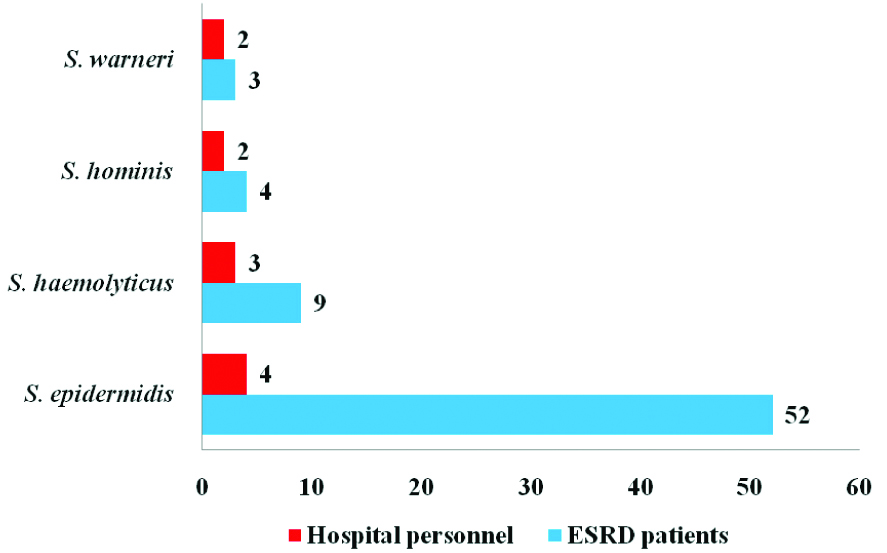
Of these MR-CoNS isolates, 68/105 (65%) were from ESRD patients and 11/30 (37%) were from hospital personnel. Among the 79 MR-CoNS isolates, S. epidermidis 56/79 (71%) was the predominant species followed by S. haemolyticus 12/79 (15%), and S. hominis 6/79 (7.5%) [Table/Fig-7].
Species distribution of MR-CoNS from ESRD patients (n=68) and Hospital personnel (n=11).
Detection of antibiotic resistant genes of conventional and newer antibiotics
All isolates exhibited 100% sensitivity to vancomycin. Overall, highest resistance was exhibited towards erythromycin (42; 53%) followed by co-trimoxazole (29; 36.7%) and tetracycline (18; 23%). Low level resistance was found towards fusidic acid (14;18%) followed by ciprofloxacin (12;14.6%) and linezolid (2; 2.5%) [Table/Fig-8]. Erythromycin resistance was found in 42 isolates (53%), of which 12 isolates showed iMLSB phenotype, 7 isolates exhibited cMLSB phenotype and 23 isolates exhibited MSB phenotype.
Antibiotic resistance among MR-CoNS from ESRD patients and hospital personnel.
| ESRD patients n=105 (%) | Hospital personnel n=30 (%) |
|---|
| No. of MR-CoNS isolates (n=79) | 68 (65) | 11 (37) |
| Antibiotic resistance |
| Co-trimoxazole (n=29) | 26 (38) | 3 (27) |
| Ciprofloxacin (n=12) | 8 (12) | 4 (36) |
| Erythromycin (n=42) | 35 (51) | 7 (64) |
| Fusidic acid (n=14) | 14 (21) | 0 (0) |
| Gentamycin (n=17) | 17 (25) | 0 (0) |
| Linezolid (n=2) | 2 (3) | 0 (0) |
| Tetracycline (n=18) | 13 (19) | 5 (37) |
Number in parenthesis indicates percentage
Correlation between phenotypic and genotypic traits of resistance to the antibiotics was absolute. Among the 17 isolates showing aminoglycosides resistance, 11 (64.7%) were positive for aac(6′)-Ie-aph(2″)-Ia gene, 4 (23.5%) isolates harbored aph(3′)- IIIa gene, whereas 2 (11.8%) isolates carried both aph (3′)- IIIa and ant(4′)-Ia genes. Among the 42 erythromycin resistant isolates tested for erm(A), erm(C) and msrA genes; the msrA gene (n=23; 54.7%) was the most predominant gene followed by erm(C) gene (n=14; 33.3%) and combination of both erm(A) and erm(C) gene (n=5; 12%). Two isolates which showed linezolid resistance carried cfr gene and mutation of G2576T in domain V of 23S rRNA gene.
Diversity of SCCmec and type IV subtypes
SCCmec typing was carried out for all the MR-CoNS isolates by M-PCR. Among the 79 isolates, 62 had a single SCCmec type including type I (n=18), type IV (n=27 and type V (n=15), while 17 isolates had two types [Table/Fig-9,10].
Distribution of SCCmec elements and type IV subtypes among MR-CoNS.
| SCCmec types | Total n=79 | S. epidermidis n=56 (71%) | S. haemolyticus n=12 (15%) | S. hominis n=6 (7.5%) | S. warneri n=5 (6.5%) |
|---|
| Type I | 18 | 10 (13) | 4 (5) | 0 (0) | 4 (5) |
| Type II | 2 | 1 (1.2) | 0 (0) | 1 (1.2) | 0 (0) |
| Type IV | 27 | 21 (26.5) | 3 (3.7) | 2 (2.4) | 1 (1.2) |
| Type V | 15 | 8 (10) | 5 (6.3) | 2 (2.4) | 0 (0) |
| Type I+V | 8 | 7 (8.8) | 0 (0) | 1 (1.2) | 0 (0) |
| Type IV+III | 6 | 6 (7.5) | 0 (0) | 0 (0) | 0 (0) |
| Type II+V | 3 | 3 (3.7) | 0 (0) | 0 (0) | 0 (0) |
| SCCmec type IV subtyping (n=27) |
| Type IVA (n=21) | 21 | 16 (59) | 3 (11.1) | 2 (7.4) | 0 (0) |
| Type IVG (n=2) | 2 | 2 (7.4) | 0 (0) | 0 (0) | 0 (0) |
| Type IVD (n=1) | 1 | 1 (3.7) | 0 (0) | 0 (0) | 0 (0) |
| Non-typable (n=3) | 3 | 2 (7.4) | 1 (3.7) | 0 (0) | 0 (0) |
‘n’ indicates no of isolates and figures in parenthesis indicates percentage
Analysis of antibiotic resistance genes and its associated SCCmec types.
| SCCmec types | Antibiotic resistant determinants |
|---|
| aac (6´)-Ie-aph (2´)-Ia | aph (3´)-IIIa | tetK | msrA | erm(C) | dfrA | fusB | fusC |
|---|
| Type I (n=18) | 4 (22%) | 3 (17%) | 3 (17%) | 11 (61%) | 3 (17%) | 12 (67%) | 4 (22%) | 2 (11%) |
| Type II (n=2) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Type IV (n=27) | 1 (3.7%) | 0 (0) | 4 (14.8%) | 5 (18.5%) | 6 (22.2%) | 8 (29.6%) | 1 (3.7%) | 1 (3.7%) |
| Type V (n=15) | 3 (20%) | 0 (0) | 4 (27%) | 4 (27%) | 3 (20%) | 4 (27%) | 0 (0) | 0 (0) |
| Type I+V (n=8) | 0 (0) | 0 (0) | 0 (0) | 2 (25%) | 0 (0) | 1 (12.5%) | 2 (25%) | 2 (25%) |
| Type III+IV (n=6) | 2 (33.3%) | 2 (33.3%) | 4 (67%) | 0 (0) | 2 (33.3%) | 3 (50%) | 1 (17%) | 0 (0) |
| Type II+V (n=3) | 1 (33.3%) | 0 (0) | 3 (100%) | 0 (0) | 0 (0) | 1 (33.3%) | 0 (0) | 1 (33.3%) |
| Total | 11 | 5 | 18 | 23 | 14 | 29 | 8 | 6 |
Antibiotic resistant genes and its associated SCCmec types
An analysis of the resistant genes of different SCCmec types among the MR-CoNS showed that resistance to non-β-lactam antibiotics was more common in SCCmec type I and SCCmec type IV, the most frequently identified SCCmec type which also exhibited high rates of occurrence of all the resistant genes [Table/Fig-9,10].
Discussion
ESRD patients receiving haemodialysis will be in constant flux with both the hospital and community environment and have a significantly higher risk of colonisation by invasive staphylococcal population than normal population. The spread of MR-CoNS from hospital settings has been reported in several recent studies. However, studies on the antibiotic resistance genes and diversity of MR-CoNS in ESRD patients are currently very scarce. Here, we studied the antibiotic resistance genes and diversity of SCCmec elements of MR-CoNS from ESRD patients and hospital personnel. The data regarding high level mupirocin resistance among CoNS from nasal carriers of these study subjects has already been reported and published as a correspondence by the same research group [25].
Species distribution of MR-CoNS showed that S. epidermidis was the predominant species which was in accordance with the previous study [5,26]. Increasing resistance of MR-CoNS to antimicrobial agents and the worldwide emergence and spread of MR-CoNS in particular is of deep concern. The results of this study indicate a higher rate of MR-CoNS (65%) among carrier isolates obtained from haemodialysis patients compared to other reports of MR-CoNS [12]. Higher isolation rate of MR-CoNS was also accompanied by higher resistance to other classes of antibiotics including erythromycin (42;53%), co-trimoxazole (29;36.7%), tetracycline (18;23%), fusidic acid (14;18%) and linezolid (2; 2.5%).
Among aminoglycosides resistant genes, aac (6′)-Ie-aph(2″)-Ia was the most prevalent gene (64.7%) which was in agreement with the previous reports [26-28]. Among isolates showing macrolide and tetracycline resistance, msrA gene (58.7%) and tet(K) gene (22%) were the most prevalent genes in this study which was in agreement with the other studies whereas tetM was not found in any of the isolates unlike previous reports in which both tetK and tetM genes were found [26,27]. Fusidic acid resistance was low (18%) which was significantly lower than the previous study [20].
In the present study, we found two isolates (one S. epidermidis and one S. haemolyticus) harbouring cfr gene and G2576T point-specific mutation within the domain V of 23S rRNA. The clinical history of the patient revealed prior exposure to linezolid for prophylaxis, which might have preceded successful colonisation of linezolid resistant strains. In particular, two linezolid resistant isolates carried six antimicrobial resistance genes that confer resistance to five different antibiotics. The mutation occurring in 23S rRNA in staphylococci leads to resistance which is non-transferable. Even though we could control the dissemination of 23S rRNA gene by following strict infection control practices in health care settings, it is impossible to control the dissemination of cfr gene mediated resistance due to the fact that this gene is transferred horizontally across bacterial species. There is a need to emphasise the rational antibiotic use and keep linezolid as a reserve because this linezolid drug can be easily exploited in clinical practice by administering orally for treating staphylococcal infections.
In agreement with the previous reports, SCCmec elements were more diverse among MR-CoNS isolates [5,10,11,28,29]. This study revealed great diversity of SCCmec among 79 MR-CoNS isolates in which 62/79 (78.5%) isolates harbored single type and 18/79 (22.3%) isolates harbored two types of SCCmec elements. Among S. epidermidis (n=56), more diversity of SCCmec types was seen of which, SCCmec type IV (n= 21) was the predominant type, followed by type I (n= 10), type V (n= 8) and 16 isolates showed combination of two types. Overall, we observed decreasing prevalence of SCCmec types II, III and V and an increasing prevalence of SCCmec types IV and V. SCCmec IV is reportedly the most frequently acquired SCCmec by S. epidermidis, which is in accordance with the enhanced mobility of this type of SCCmec observed in S. aureus [10,26,28].
The high number of different SCCmec types present in MR- CoNS can build up a large reservoir of new SCCmec types for S. aureus and probably facilitate horizontal transmission among staphylococcal species. It is revealed that the presence of two SCCmec elements appears to be common among MR-CoNS which strongly suggest that new variants may be present in MR-CoNS, which can have a major influence in acquiring resistance to the drugs [10,11,29]. The ESRD patients warranting long term hospitalisation with constant community interactions might act as a reservoir for the dissemination of such acquired resistant determinants.
Limitation
The limitation in our study is the lack of prevalence data on nasal colonisation and antibiotic resistance of MR-CoNS from the hospital as no data was available. The other limitation in this study is that currently we do not have follow-up data regarding the patient cohort for endogenous infections. As it is an ongoing study, we are following up with our patient cohort for endogenous infection and we intend to publish a manuscript on that soon.
Conclusion
The findings of our study substantiate the need for minimising nasal colonisation of MR-CoNS which may act as a reservoir for endogenous infections in ESRD patients. There is an unmet need for continuous monitoring of antibiotic susceptibility pattern of all MR-CoNS isolates for selection of appropriate therapy.
SD: Standard deviation