Serological Diagnosis of Indian Tick Typhus in and around Puducherry: Application of Indirect Immunofluorescence Assay
Selvaraj Stephen1, Dhandapany Gunasekaran2, Jothimani Pradeep3, Stanley Ambroise4, Kengamuthu Sarangapani5
1 Professor, Department of Microbiology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth (Deemed-to-be-University), Pillaiyarkuppam, Puducherry, India.
2 Professor, Department of Paediatrics, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth (Deemed-to-be-University), Pillaiyarkuppam, Puducherry, India.
3 Ph.D. Scholar, Department of Microbiology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth (Deemed-to-be-University), Pillaiyarkuppam, Puducherry, India.
4 Specialist Grade I, Department of General Medicine, Indira Gandhi Government General Hospital and Post Graduate Institute, Puducherry, India.
5 Specialist Grade I, Department of Microbiology, Indira Gandhi Government General Hospital and Post Graduate Institute, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Selvaraj Stephen, Professor, Department of Microbiology, Mahatma Gandhi Medical College and Research Institute, Pondy-Cuddalore Main Road, Pillaiyarkuppam-607403, Puducherry, India.
E-mail: stephens4950@gmail.com
Introduction
In the past, tick typhus caused by Rickettsia conorii has been reported from different parts of India. However, during the last two decades, reports are few and far between with only some case reports and very few seroprevalence studies.
Aim
To estimate the seroprevalence of Indian tick typhus among febrile patients of Puducherry and surrounding Tamil Nadu, employing Indirect Fluorescence Antibody (IFA) test, which is considered as the ‘gold standard’ serological test for diagnosing rickettsial diseases.
Materials and Methods
This study was conducted during the period February 2018 to March 2019 at Mahatma Gandhi Medical College and Research Institute and Indira Gandhi Government General Hospital and Post Graduate Institute, Puducherry, India. The study included 114 febrile patients who provided both acute and convalescent serum samples. Forty ante-natal women and 23 voluntary blood donors were included as healthy controls. All sera were examined for IgG antibodies to Rickettsia conorii by IFA test (Fuller Laboratories, Fullerton, California, USA). Mean±Standard deviation with 95% confidence interval was calculated for age of the patients and duration of illness using Graph Pad Quick Calcs Software, USA. Chi-square and Fisher’s-exact tests were performed and p-value ≤0.05 was considered as statistically significant.
Results
Out of 114 febrile patients screened for Spotted Fever (SF) IgG IFA, 27 were positive in IgG IFA with titres ranging from 1:128 to 1:2048 (23.68%). Among the control group (n=63), only one participant was seropositive for R. conorii IgG IFA (1.59%). Statistical difference in seropositivity between the febrile patients and healthy controls was quite significant (p=0.0001).
Conclusion
Due to large number of false positivity, presence of IgM antibodies in acute SF is of doubtful significance. Presence of significant titres of Spotted Fever Group (SFG) IgG antibodies in IFA (≥1:128) in acute serum or four fold increase in titres in paired sera are the recommendation of Centre for Disease Control and Prevention (CDC) to confirm SF.
Rickettsia conorii, Spotted fever, Tick-borne rickettsioses, Zoonosis
Introduction
Infection caused by Rickettsia conorii is a zoonotic, worldwide emerging infectious disease and categorised under Spotted Fever Group (SFG) rickettsioses. The disease is known by different names such as Boutonneuse fever, Indian tick typhus and Mediterranean spotted fever [1-3]. The knowledge regarding the prevalence of SFG in India and other developing countries is inadequate [4,5]. Indian tick typhus and Scrub typhus are endemic in India and/are closely related to each other in their clinical presentation [6,7]. Disease can be transmitted to humans by the bite of brown dog tick Rhipicephalus sanguineus. Initially the symptoms can start with 6-10 days of fever, rash, lymphadenopathy, muscle pain, headache and now eschar has also been recently added to these features [1,2]. Rash may be small, macular on ankle joints or forearms and spreading to legs, trunk palms, soles and face [8]. Rash is one of the most important predictor of rickettsial disease but unfortunately, more than 50% of the suspected rickettsial patients have no rash during the window period [1,9,10]. Isolation is restricted to reference laboratories only which have Bio-safety level III containment facilities [1]. Recently there is a spurt of SF cases presenting with haemorrhagic rashes, cardiac involvement, retinitis, tubercular meningitis and Meningoencephalitis [7,9-14]. Serological diagnosis of SF was made on the basis of the results of tests like Weil-Felix reaction [15-20], Enzyme Linked Immunosorbent Assay (ELISA) [21-25] and Indirect Immuno Fluorescence Assay (IFA) [11,15,24,26]. Usually, the evidence of SFG is confirmed by serological tests and the antibodies will appear on second week of illness [1,2]. Molecular tests are highly helpful in early detection of specific SFG DNA by targeting various conserved genes viz., 16 srRNA, gltA, 17 kDa, Omp A, Omp B [27]. To the best of our knowledge this is the first study from Puducherry highlighting the serological evidence of R. conorii using the ‘gold standard’ IFA test. Our main objective was to understand the true prevalence of SFG infection among febrile patients and compare with normal population comprising of healthy pregnant women and voluntary blood donors.
Materials and Methods
This was a prospective and laboratory based study, which was conducted during the period February 2018 to March 2019 in the Department of Microbiology, Mahatma Gandhi Medical College and Research Institute and Indira Gandhi Government General Hospital and Post Graduate Institute, Puducherry, India. The work started after getting approval from our Institutional Human Ethics Committee (IHEC) (FACULTY/12/2017/28 dated 10/12/2017). A total of 114 paired serum samples were collected from the febrile patients comprising of both acute and convalescent samples taken at intervals of two to three weeks. Forty sera from healthy ante-natal women and 23 samples from voluntary blood donors were included as controls. Inclusion and exclusion criteria were as reported earlier [25].
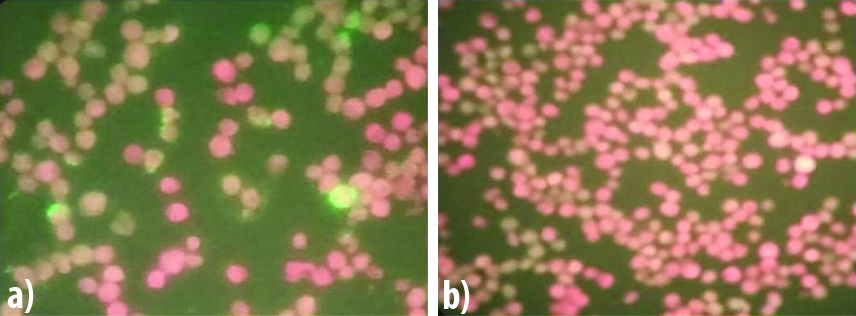
The clinical features, demographic details and laboratory results were retrieved from the patients’ case records. All sera were subjected to SF Rickettsia conorii IgG IFA test (Fuller Laboratories, Fullerton, California, USA). The procedure was strictly carried out as per the manufacturer’s instructions. Briefly, the serum samples were diluted 1:64 with Phosphate Buffered Saline (PBS). Ten microlitres of 1:64 diluted samples including the positive and negative controls provided in the kit were added to the antigen spots. The slides were coated with killed and acetone fixed Vero cells some of which were infected with Moroccan strain of R. conorii. Slides were kept in a humidity chamber and incubated at 37°C for 30 minutes, followed by washing with gentle stream of PBS for three times and then placed in a beaker containing PBS and magnetic beads and washed gently for five minutes. Slides were removed, dried and 15 μL of conjugate (affinity purified FITC-labeled goat anti-human IgG (heavy chain) with bovine serum albumin and Evans’ blue counterstain) was added to the wells and further incubated in dark in a humidity chamber at 37°C for 30 minutes. After incubation, the slides were again washed as before, dried, a coverslip of 24×50 mm was placed over the smears and mounted using mounting medium. Slides were observed at 400X magnification of the fluorescent microscope (Primo Star iLEDZeiss Microscope, Germany). The positive reactions showed short pleomorphic rods with apple green fluorescence and negative reaction shows red fluorescence [Table/Fig-1]. The smears were scored as 1+ to 4+. According to the technical brochure of the kit, the significant titre being ≥1:64.
Images of SF R. Conorii IgG IFA. a) Positive Control (≥1:128); b) Negative control.
Statistical Analysis
Mean±Standard deviation with 95% confidence interval for the age and duration of illness of the patients who were positive and negative for R. conorii IgG antibodies was calculated using Graph Pad Quick Calcs Software, USA. Chi-square and Fisher’s-exact tests were performed for numerical variables and p-value ≤0.05 was considered as statistically significant.
Results
Out of 114 febrile patients who provided paired sera and screened for SF IgG IFA, 27 were positive in IgG IFA with titres ranging from 1:128 to 1:2048 (23.68%). Among the control group (n=63), only one participant was seropositive for R. conorii IgG IFA (1.59%). Statistical difference in seropositivity between the febrile patients and healthy controls was quite significant (p=0.0001). While all the voluntary blood donors (n=23) were seronegative, a single ante-natal mother among 40 women was seropositive (2.5%). SF seropositive patients were aged between 9 to 60 years and the mean and SD age with 95% confidence interval was 33.96 (SD=13.76) (28.77-39.15) for R. conorii IgG antibodies positive patients. Among 27 seropositive patients, 16 were females and 11 were males. However, there was no statistical difference between the two sexes (p=0.156). Eleven patients had seroconversion upto titres of 1:128/1:256. Three patients showed four-fold (1:512) and another patient sixteen-fold increase (1:2048) in the convalescent sera. Only one ante-natal woman had significant titer of 1:128 [Table/Fig-2]. None of the voluntary blood donors were positive for R. conorii IgG antibodies. [Table/Fig-3] shows the details of clinical and laboratory parameters, which are statistically significant: duration of illness (p=0.0111), chills, rigor (p=0.0388) and thrombocytopenia (p=0.0079). Other clinical and laboratory parameters like presence of eschar, lymphadenopathy, headache, myalgia and other gastrointestinal symptoms were of no statistical significance. Two patients developed complication with acute renal failure and one Myocarditis. Two patients gave a history of contact with domestic animals like dog/goat/cat.
R. conorii IgG IFA titres in paired samples of patients (n=27).
| S. No. | Age/Sex | Days of fever | Titres of Paired serum samples |
|---|
| A | C |
|---|
| 1 | 37/F | 4 | Neg | 1:128 |
| 2 | 40/F | 10 | 1:128 | 1:128 |
| 3 | 41/M | 7 | Neg | 1:128 |
| 4 | 49/F | 15 | 1:128 | 1:2048 |
| 5 | 23/F | 10 | 1:128 | 1:256 |
| 6 | 60/M | 21 | Neg | 1:128 |
| 7 | 43/M | 10 | 1:256 | 1:512 |
| 8 | 37/F | 10 | 1:256 | 1:128 |
| 9 | 20/F | 7 | 1:128 | Neg |
| 10 | 9/F | 11 | 1:256 | Neg |
| 11 | 24/F | 30 | 1:128 | Neg |
| 12 | 59/M | 10 | 1:128 | Neg |
| 13 | 38/M | 10 | 1:256 | 1:256 |
| 14 | 45/F | 5 | 1:128 | 1:512 |
| 15 | 27/F | 15 | Neg | 1:128 |
| 16 | 12/M | 5 | Neg | 1:256 |
| 17 | 20/F | 7 | 1:512 | 1:512 |
| 18 | 32/F | 10 | Neg | 1:256 |
| 19 | 40/F | 7 | Neg | 1:128 |
| 20 | 17/M | 7 | 1:256 | 1:256 |
| 21 | 29/M | 1 | 1:256 | 1:128 |
| 22 | 55/M | 8 | 1:128 | 1:128 |
| 23 | 30/F | 7 | 1:256 | 1:128 |
| 24 | 48/M | 5 | Neg | 1:128 |
| 25 | 30/F | 10 | Neg | 1:256 |
| 26 | 46/M | 8 | Neg | 1:128 |
| 27 | 12/F | 6 | Neg | 1:128 |
A: Acute samples; C: Convalescent samples
Clinical and laboratory parameters of febrile patients (n=114).
| Clinical/Laboratory findings | SF IFA positive patients (n=27) | SF IFA Negative patients (n=87) | Total (%) | p-value* (Chi-square test) |
|---|
| Male | 11 | 49 | 60 (52.63%) | 0.1566 |
| Female | 16 | 38 | 54 (47.36%) | 0.1566 |
| <7 days of fever | 20 | 38 | 58 (50.88%) | 0.0111 |
| >7 days of fever | 7 | 49 | 56 (49.12%) | 0.0111 |
| Headache | 12 | 30 | 42 (36.84%) | 0.3485 |
| Chills and Rigor | 16 | 32 | 48 (42.10%) | 0.0388 |
| Myalgia | 8 | 19 | 27 (23.68%) | 0.5668 |
| Malaise | 4 | 10 | 14 (12.28%) | 0.7380 |
| Nausea | 6 | 12 | 18 (15.79%) | 0.4549 |
| Vomiting | 10 | 17 | 27 (23.68%) | 0.1076 |
| Abdominal pain | 5 | 7 | 12 (10.52%) | 0.1515 |
| Cough | 6 | 14 | 20 (17.54%) | 0.6585 |
| Rash | 1 | 3 | 4 (3.50%) | 1.0000 |
| Eschar | 2 | 1 | 3 (2.63%) | 0.1392 |
| Lymphadenopathy | 2 | 5 | 7 (6.14%) | 0.6686 |
| Hepto-splenomegaly | 10 | 33 | 43 (37.72%) | 0.9333 |
| Increased liver enzymes (ALT/ALP/AST)£ | 10 | 29 | 39 (34.21%) | 0.9027 |
| Thrombocytopenia (mm3/lakhs) | 16 | 25 | 41 (35.96%) | 0.0079 |
| Complications** | 1 | 3 | 4 (3.50%) | 1.0000 |
*p≤0.05 is considered to be statistically significant
**Two patients had Acute Renal Failure and one patient had myocarditis
£AST - Aspartate Transaminase/ALT - Alanine Transaminase/ALP - Alkaline Phosphatase
Discussion
For the diagnosis of SF IgM antibodies in acute samples are not confirmatory, since false positives are too many with the persistence of IgM for a longer time and hence many authors recommend use of SF IgG IFA only [1-3,28,29]. Seropositivity only in the acute sample is not being helpful in the diagnosis of SFG. According to CDC and Council of State and Territorial Epidemiologists (CSTE) case definition has two categories viz., probable or confirmed cases [2].
Clinical Criteria
Febrile patient with one or more of the following symptoms: rash, eschar, headache, myalgia, anaemia, decreased platelets, or elevated hepatic transaminase.
Laboratory Confirmed Spotted Fever
Fourfold increase in specific IgG titer between acute and convalescent sera in IFA.
Presence of SFG Rickettsial DNA in suspected clinical samples in PCR.
Presence of SFG antigen in biopsies (by Immunohistochemistry).
Isolation of SFG Rickettsia.
Laboratory Supportive Spotted Fever
Elevation of IgG or IgM to SFG by IFA, ELISA or Dot-ELISA.
Probable case: Clinical symptoms with laboratory supportive results and
Confirmed case: Clinical symptoms with laboratory confirmed results.
Since ELISA is a qualitative test, the increase in titres between acute and convalescent samples can be inferred by performing the IFA test, which is quantitative. In the present study, 27 febrile patients were seropositive for R. conorii IgG antibodies by the serological ‘gold standard’ IFA. Among them, 13 belonged to the CDC category of confirmed SF (based on clinical evidence and laboratory confirmation). Another 14 patients come under the CDC category of probable cases of SFG (with clinical evidence and laboratory supportive results). [Table/Fig-2] shows the results of IgG IFA titers of 27 febrile patients.
Seroprevalence of SF
In USA, the prevalence of SFG was relatively high (15.0%) among aged persons from 60-69 years compared to children of <10 years (3.81%) [2]. However, our findings are different with only one patient was in the age of 60 and four children were aged between 1-18 years. Remaining 22 belonged to the age group of 19-59 years. Kantsø B et al., reported that the positivity for SF was 56% in both children and adults [29]. However, in the present study a moderate sero positivity of 23.68% was observed in our patients (both children and adults). About 1% was positive for R. conorii IgG antibodies in blood donors in Netherlands [29], but in our study none of them were positive. Rickettsial diseases are endemic in Sri Lanka and a prevalence of R. conorii infection of 40.9% using IgG IFA was reported by Kularatne SAM et al., [30]. Several researchers who documented the prevalence of SFG from various places of India employed the non-specific Weil-Felix test with a cut-off titer of ≥1:80 [15-20,25]. In a study conducted in Bangalore, 31.0% of children had SF infection with meningoencephalitis, while in the same state, another study reported 26.35% sero positivity among febrile children [15,19]. Similarly, Puducherry recorded SF sero prevalence of 21.25% [25] while Tamil Nadu showed the least positivity of 4.6% [21] all these results were obtained by the application of Weil-Felix. In Uttar Pradesh 37.1% were sero positive for Indian tick typhus in IgM ELISA [22] whereas 40.8% in children from Puducherry were positive in IgM and/or IgG ELISA [25].
Regarding adults, 17.1% were seropositive in Uttar Pradesh for R. conorii IgM by both ELISA and IFA [24] while 46.6% from Puducherry [25] were seropositive for SFG IgM/IgG ELISA.
In the current study we observed a moderate seropositivity of 23.68% among febrile patients for R. conorii IgG antibodies in IFA. The cut-off titer considered by us was ≥1:128, which has been endorsed by several researchers from India and abroad [10-14,22-24,26]. Somashekar HR et al., from Tamil Nadu reported seroprevalence of 32.6% [20] whereas in much contrast, only 2.1% seropositivity was observed in Karnataka [26] both by the application of IgM IFA.
Limitation
We could not perform molecular tests for the detection of specific gene targets. Construction of a phylogenetic tree, might throw some light regarding circulation of any new species of SFG rickettsiae other than R.conorii in this part of Southern India.
Conclusion
Present study has established a moderate seroprevalence of Indian tick typhus (23.68%) in Puducherry and surrounding Tamil Nadu, by the application of R. conorii IgG, which is considered as a specific serological test, superior to IgM IFA.
A: Acute samples; C: Convalescent samples
*p≤0.05 is considered to be statistically significant
**Two patients had Acute Renal Failure and one patient had myocarditis
£AST - Aspartate Transaminase/ALT - Alanine Transaminase/ALP - Alkaline Phosphatase
[1]. Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS, Diagnosis and management of tick borne rickettsial diseases: Rocky mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis- United StatesMMWR Recomm Rep 2016 65(2):1-44.10.15585/mmwr.rr6502a127172113 [Google Scholar] [CrossRef] [PubMed]
[2]. Centers for Disease Control and Prevention. Spotted Fever Rickettsiosis (Rickettsia spp.) 2010 Case Definition 2010 [cited 2017 September 4, 2017]. Available from: https://wwwn.cdc.gov/nndss/conditions/spotted-fever-rickettsiosis/case-definition/2010/ [Google Scholar]
[3]. Madeddu G, Fiore V, Mancini F, Caddeo A, Ciervo A, Babudieri S, Mediterranean spotted fever-like illness in Sardinia, Italy: A clinical and microbiological studyInfection 2016 44(6):733-38.10.1007/s15010-016-0921-z27380385 [Google Scholar] [CrossRef] [PubMed]
[4]. Batra HV, Review Spotted fevers & typhus fever in Tamil NaduIndian J Med Res 2007 126:101-03. [Google Scholar]
[5]. Rathi N, Rathi A, Review Rickettsial infections: Indian perspectiveIndian Pediatr 2010 47:157-64.10.1007/s13312-010-0024-320228429 [Google Scholar] [CrossRef] [PubMed]
[6]. Rathi N, Kulkarni A, Yewale V, IAP Guidelines on rickettsial diseases in childrenIndian Pediatr 2017 54:223-29.10.1007/s13312-017-1035-0 [Google Scholar] [CrossRef]
[7]. Lunge SB, Patil V, Ambar S, Naik V, Malignant Mediterranean spotted feverIndian Dermatol Online J 2015 6:1-4.10.4103/2229-5178.17105026904440 [Google Scholar] [CrossRef] [PubMed]
[8]. Rahi M, Gupte MD, Bhargava A, Varghese GM, Arora R, DHR-ICMR Guidelines for diagnosis & management of Rickettsial diseases in IndiaIndian J Med Res 2015 141:417-22.10.4103/0971-5916.15927926112842 [Google Scholar] [CrossRef] [PubMed]
[9]. Mahto SK, Gupta PK, Sareen S, Balakrishna AM, Suman SK, A case of rocky mountain spotted fever without eschar as a cause of pyrexia with multiple organ failureInt J Res Med Sci 2017 5:4658-60.10.18203/2320-6012.ijrms20174618 [Google Scholar] [CrossRef]
[10]. Shriyan A, Ashvij S, An atypical presentation of Rocky Mountain Spotted Fever (RMSF)-A Case ReportJ Clin Diagn Res 2010 4:2546-49. [Google Scholar]
[11]. Shah V, Vaidya V, Bang V, Shah I, Spotted fever in a child in Mumbai, IndiaJ Vector Borne Dis 2009 46:310-12. [Google Scholar]
[12]. Sundhindra BK, Vijayakumar S, Kutty KA, Tholpadi SR, Rajan RS, Mathai E, Rickettsial spotted fever in KeralaNatl Med J India 2004 17:51-52. [Google Scholar]
[13]. Khan SA, Bora T, Ahmed S, Malang SMIS, Devi U, Kakati S, Spotted fever rickettsiae and tuberculous meningitis dual infection presentingas acute encephalitis syndrome: A fatal case reportJ Vector Borne Dis 2017 54:194-96. [Google Scholar]
[14]. Sarma A, Rickettsia (Spotted Fever Group) infection with multiorgan dysfunctionAmerican Journal of Medical Case Reports 2015 3:322-24. [Google Scholar]
[15]. Reddy BK, Basavaraja GV, Rickettsial Meningoencephalitis: An under diagnosed entity in developing countriesJournal of Pediatric Sciences 2013 5:e119310.1016/j.pid.2013.03.005 [Google Scholar] [CrossRef]
[16]. Mahajan SK, Kashyap R, Sankhyan N, Sharma V, Rolain JM, Prasher BS, Spotted fever group rickettsioses in Himachal PradeshJ Assoc Physicians India 2007 55:868-70. [Google Scholar]
[17]. Murali N, Pillai S, Cherian T, Raghupathy P, Padmini V, Mathai E, Rickettsial infections in south India-how to spot the spotted feverIndian Pediatrics 2001 38:1393-96. [Google Scholar]
[18]. Vinoth S, Prabhakaran A, Lal S, Murali V, Sankar G, Jayabalan N, Outbreak of scrub typhus and spotted fever group in human and rodent populations in Kolar, South IndiaArch Clin Microbiol 2011 2:1-7. [Google Scholar]
[19]. Wadekar MD, Rani NBS, Seroprevalence of rickettsial diseases in a tertiary care hospitalInt J Curr Microbiol App Sci 2016 5:14-18.10.20546/ijcmas.2016.509.002 [Google Scholar] [CrossRef]
[20]. Somashekar HR, Prabhakar DM, Sreeja P, Elizabeth M, Didier R, Jean MR, Magnitude and features of scrub typhus and spotted fever in children in IndiaJ Trop Pediatr 2006 52:22910.1093/tropej/fmi09616291832 [Google Scholar] [CrossRef] [PubMed]
[21]. Kamarasu K, Malathi M, Rajagopal V, Subramani K, Jagadeeshramasamy D, Mathai E, Serological evidence for wide distribution of spotted fevers & typhus fever in Tamil NaduIndian J Med Res 2007 126:128-30. [Google Scholar]
[22]. Kalal BS, Puranik P, Nagaraj S, Rego S, Shet A, Scrub typhus and spotted fever among hospitalised children in South India: Clinical profile and serological epidemiologyIndian J Med Microbiol 2016 34:293-98.10.4103/0255-0857.18831527514949 [Google Scholar] [CrossRef] [PubMed]
[23]. Singh M, Agarwal J, Tripathi CDP, Kanta C, Spotted fever rickettsiosis in Uttar PradeshIndian J Med Res 2015 141:242-44.10.4103/0971-5916.15559625900962 [Google Scholar] [CrossRef] [PubMed]
[24]. Tripathi CDP, Singh M, Agarwal J, Kanta C, Atam V, Seroepidemiology of spotted fever rickettsiosis in Uttar Pradesh: A prospective studyJ Clin Diagn Res 2017 11:DC04-09.10.7860/JCDR/2017/25926.1002928764157 [Google Scholar] [CrossRef] [PubMed]
[25]. Stephen S, Ambroise S, Gunasekaran D, Hanifah M, Sangeetha B, Pradeep J, Serological evidence of Spotted Fever Group rickettsiosis in and around Puducherry, South India-A three years studyJ Vect Borne Dis 2018 55:144-50.10.4103/0972-9062.24256230280713 [Google Scholar] [CrossRef] [PubMed]
[26]. Koraluru M, Bairy I, Varma M, Athan E, Stenos J, Spotted fever group and typhus fever group rickettsiosis in South Western IndiaInt J Infect Dis 2016 45S:180-81.10.1016/j.ijid.2016.02.423 [Google Scholar] [CrossRef]
[27]. Prakash JA, Sohan LT, Rosemol V, Verghese VP, Pulimood SA, Reller M, Molecular detection and analysis of spotted fever group Rickettsia in patients with fever and rash at a tertiary care centre in Tamil Nadu, IndiaPathog Glob Health 2012 106:40-45.10.1179/2047773212Y.000000000122595273 [Google Scholar] [CrossRef] [PubMed]
[28]. McQuiston JH, Wiedeman C, Singleton J, Carpenter LR, McElroy K, Mosites E, Inadequacy of IgM antibody tests for diagnosis of rocky mountain spotted feverAm J Trop Med Hyg 2014 91:767-70.10.4269/ajtmh.14-012325092818 [Google Scholar] [CrossRef] [PubMed]
[29]. Kantsø B, Svendsen CB, Jørgensen CS, Krogfelt KA, Evaluation of serological tests for the diagnosis of rickettsiosis in DenmarkJ Microbiol Methods 2009 76:285-88.10.1016/j.mimet.2008.12.01219162092 [Google Scholar] [CrossRef] [PubMed]
[30]. Kularatne SAM, Rajapakse RPVJ, Wickramasinghe WMRS, Nanayakkara DM, Budagoda SS, Weerakoon KGAD, Rickettsioses in the central hills of Sri Lanka: serological evidence of increasing burden of spotted fever groupInt. J Infect Dis 2013 17:e988-92.10.1016/j.ijid.2013.05.01423871280 [Google Scholar] [CrossRef] [PubMed]