Globally, breast cancer is the most frequent malignancy and the foremost reason of cancer mortality in females. In Iraq, breast cancer is the most common type of malignancy in females after cardiovascular diseases, accounting for approximately one-third of the registered female cancers according to the Iraqi Cancer Registry [1,2]. Many factors control the risk for breast cancer, and the most important of these related to diet, drugs, and the levels of oestrogen, androgens and prolactin. One risk factor that is variable is obesity, mainly in the postmenopausal woman [3].
Generally, most of the chemotherapeutics like: methotrexate, 5-fluorouracil showed a narrow therapeutic index [4]. Current drug discovery and studies are focused on increasing the therapeutic efficiency of chemotherapeutics at low dose. To achieve this goal, scientists require pre-clinical models that can dependably conceal anti-cancer agents with robust clinical correlation [5]. Remarkably, doxorubicin (DOX) and 5 fluorouracil (5-FU) have been reported to induce apoptosis in the human breast cancer cell line MCF-7 [6-8]. DOX, an anthracycline antibiotic, is one of most common chemotherapeutics with a wide anti-cancer spectrum [9], but its clinical use is still restricted by the severe side effects such as cardiotoxicity as well as common side effects such as: nausea and vomiting, diarrhea, loss of appetite, tiredness, eye redness [10,11]. DOX intercalates into DNA and stops the topoisomerase II progression which ends the DNA replication [10]. The 5-Fluorouracil (5-FU) is a thymidylate synthase inhibitor that prevents the production of deoxythymidine monophosphate (dTMP) and causing rapidly dividing cells to end the replication and then the cells to die [12]. Using of 5 FU as a typical chemotherapeutic agent has been effectively proved in many of cancers including colorectal and breast [13-15]. In breast cancer treatment, the combination therapy such as: Adriamycin and Cytoxan (AC), Adriamycin and Taxotere (AT): Cytoxan, methotrexate, and fluorouracil CMF has been the standard of care, the advantages of the combination chemotherapy are; improved patient compliance, emergence of interaction effects such as synergistic or additive, ability to overcome Multi Drug Resistance (MDR) and decrease of drug doses with high toxicity to normal tissues [16]. The common problems related with mono-therapy involve drug resistance at tumour and cellular levels that delay drug diffusion and effectiveness, therefore the drug combination studies on tumour cell lines by using a quantitative method to assess the nature of drug interactions allow more reasonable design of forthcoming therapy protocols [17].
An early evidence of preclinical study model by Liu Z et al., exposed that even intratumoural co-administration of DOX and a chemosensitizer of P-gp could only generate modest effect on tumour growth delay while noticeable toxicity was observed in MDR breast tumour bearing murine model [18].
Increasing interest in drug combinations might inhibit cancer as summarised in Nature article by Gravitz I [19]. In this study, we have studied the rationale and advantages of applying combination therapy of doxorubicin and 5-fluorouracil to improve anticancer drug efficiency on human breast cancer cell line (MCF-7).
Materials and Methods
The study was conducted from November 2014 to June 2018, in the University of Edinburgh. All the investigations conformed to the ethics of research and the study was approved by the Ethics Committee of the Unversity.
Chemicals: Doxorubicin hydrochloride and 5-fluorouracil were purchased from molekula (UK). MTT 3-(4,5-Dimethylthiazol-2-yl)-2, 5 Diphenyltetrazolium Bromide was purchased from Fisher scientific (England). The other armamentarium and solvents used in the study were-Dulbecco’s Modified Eagle’s Medium (DMEM) medium, fetal bovine serum, phosphate buffer saline, isopropanol, trypsin, glutamine and penicillin/streptomycin, Nondet P-40 (NP40), hydrochloride acid were obtained from (Sigma Aldrich, Germany).
MTT solutions: MTT stock solution was prepared by adding (5)mg/mL of MTT in DMSO. This solution was sterilised by filtration with 0.45 μm filter unit. MTT solvent was prepared to solubilize the MTT solution by adding (800) μL of HCl (0.04 N) and 200 μL 0.1% Non det P-40 (NP40) in (14) mL of absolute isopropanol. Best grade solutions and chemicals were used in this study.
Cell culture: Human breast cancer cell line MCF-7 was obtained from American Type Culture Collection ATCC (USA) and were grown in Dulbecco’s modification of Eagle medium DMEM medium supplemented with 10% fetal bovine serum, 2 μM of L-glutamine in the presence of 1% penicillin/streptomycin. The cell culture was maintained in a 37°C incubator with a humidified 5% CO2 containing atmosphere. Cells were maintained in the logarithmic growth phase. Cells were tested regularly for mycoplasma contamination. MCF-7 (5000 cells/well) were seeded in 96 well plates and allowed to adhere overnight before exposure to drug and grown to 80-90% confluency. Then, the cell culture media were replaced with drug-containing media. The cells were exposed to drugs for 72 hours, followed by cell viability test for single and combination drug treatments as described later.
In vitro cytotoxicity: The cytotoxicity of the drugs or their combinations were evaluated by using 3-(4, 5-Dimethylthiazol-2-yl)-2, 5 Diphenyltetrazolium Bromide (MTT) viability assay [20]. The MCF-7 cells were seeded at a density of 5000 cells/well in 96-well plates and incubated for 24 hour with CO2 incubator. After 24 hours, MCF-7 cells were treated with DOX alone at different low concentrations 0.1, 0.2, 0.4, 0.6, 0.8 nM, and another MCF-7 cultured 96 well plate was treated with 5FU after 24 hours at different low concentrations 5, 10, 25, 50, 100, and 150 μM. Cell viability was determined by reading the fluorescence at 450 nm using a Modulus Microplate Reader (Turner BioSystems, USA). The cell viability was calculated using the following formula: Cell viability (%)={(absorbance of the treated cells- blank)/(absorbance of the untreated cells-blank)}*100.
The experiment was achieved with 6 replicas. The IC50 (the concentration required for 50% cell inhibition) was calculated using the GraphPad Prism 5 program (GraphPa Software Inc., USA). The combination effect proposed by Chou TC et al., [21] based on IC50 values obtained from drugs alone and their combination was evaluated by CI when MCF-7 treated with 10 μM of 5FU and different concentration of DOX 0.1,0.2,0.4,0.6,0.8,1 nM, CI was used to determine the nature of synergism, antagonism and additivity action of drug combinations as: synergistic effect is recorded if CI <1, additive effect if CI=1, or antagonism if CI >1. Dm. (the median-effect dose that inhibits 50% of the growth under study) also studied. DRI (a measure of how many-fold the dose of each drug in a synergistic combination may be reduced at a given effect level compared with the doses of each drug alone) was also calculated to indicate the level of dose reduction for each drug in synergistic combination., DRI=1 indicates no dose reduction, whereas DRI>1 and <1 indicate favourable and unfavourable dose reduction, respectively. Experiments were achieved with triplicate samples.
MCF-7 Morphological changes study: Inverted microscope (Olympus, USA) was used to study MCF-7 cells morphology, cell changes alteration was studied as follows:
MCF-7 cells were cultured in 60 mm cell culture dish, after confluence (70%-80%), cells were treated with various concentration of DOX and 5FU at IC50 and DOX/5FU for 72 hour.
The morphological changes of the apoptotic cells were examined under inverted microscope at 40x magnifications and captured by digital camera (Sony, Japan) after 72 hour of incubation, apoptotic features were identified by the manifestation of cell shrinkage and/or the presence of membrane-bound cellular bodies.
Statistical Analysis
The MTT assay data were expressed as mean±Standard Deviation (SD). The IC50 values each drug were computed by GraphPad Prism 5.2 software (GraphPad Software Inc., USA). Dose-effect curve parameters, CI values, Fa-CI, DRI-Faplot were calculated by CompuSyn program (Compusyn Inc, USA). A p-value of <0.05 represented a statistically significant difference.
Results
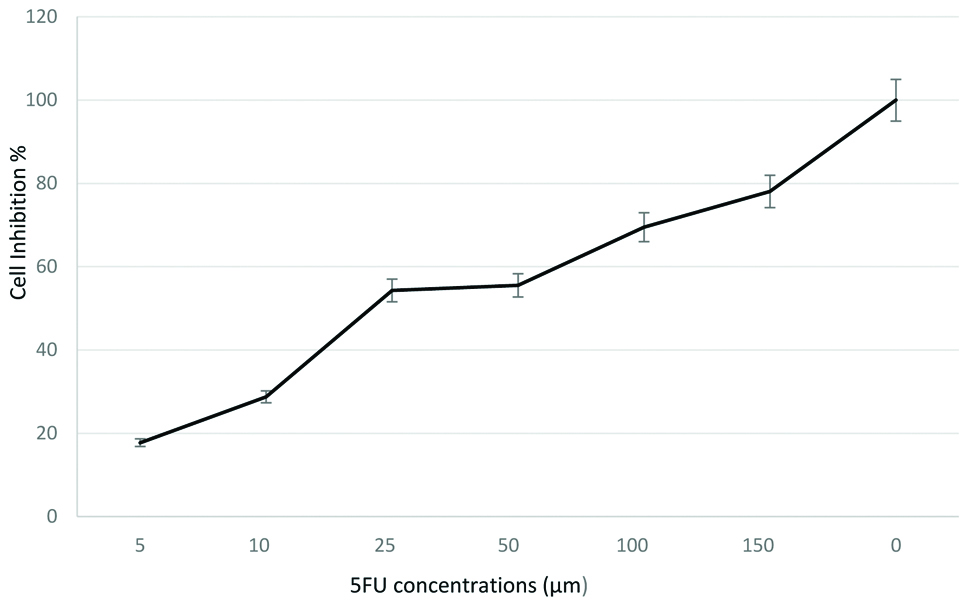
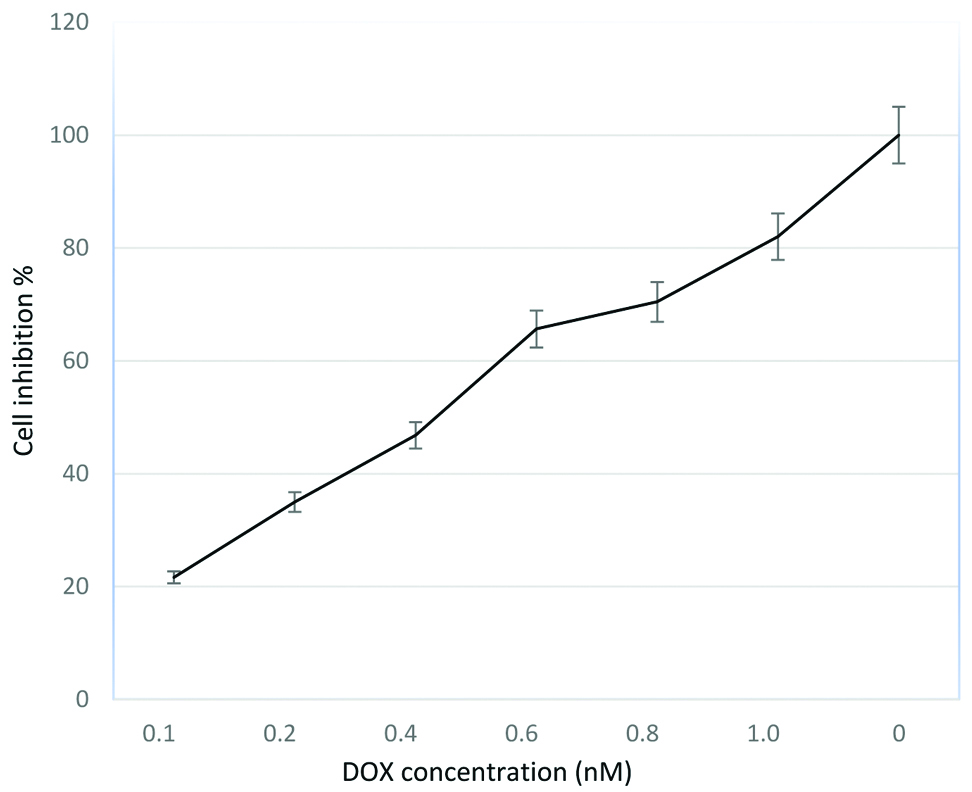
Cytotoxic effects of single drug: The cytotoxic effect of doxorubicin and 5 flurouracil was first determined independently on human breast cancer cell line MCF-7 cell line that were subjected to serial dilutions ranging from 5-150 μM of 5FU and 0.1-1 nM of DOX [Table/Fig-1,2]. Depending on the data in figures, both 5FU and DOX inhibited the proliferation of MCF-7 cells in a dose-dependent effect. IC50±SD values were 21.7±2.30 μM and 5.6±0.012 nM for 5FU and DOX respectively. Cell proliferation decreased significantly as DOX concentrations increased to 1 nM [Table/Fig-2]. These results show that the effectiveness of each drug cannot be compared directly by using the IC50 values. The cytotoxicity effect shows that MCF-7 is more sensitive to DOX than 5FU single-drug treatment. For 5FU, Dm was (20.8026±7.03) and r was (0.94164), while for DOX, Dm and r were (0.31621±0.06), (0.95935), respectively.
Dose response curves when MCF-7 cells were exposed to 5FU for 72 hours, followed by MTT assay to determine cell viability (mean±SD, n=6).
Dose response curves when MCF-7 cells were exposed to DOX for 72 hours, followed by MTT assay to determine cell viability (mean±SD, n=6).
Cytotoxic effects of two drugs in combination: To determine if DOX and 5FU drug combinations had resulted synergistic, antagonist, or additive actions, the median effect method of Chou TC et al., [19-21] was used to determine CI that is summarised in [Table/Fig-3].
The CI plot of combinations treatment for 5flourouracil and doxorubicin drugs. 5FU=5fluorouracil, DOX=doxorubicin.
Data points represent (the average±standard deviation), CI values <1 are symbolic of synergistic effects; CI values >1 are symbolic of antagonistic effects; and CI values =1 are symbolic of additive effects, Fraction Affected (FA) is a measure of the determined effect (cytotoxicity as measured by an MTT assay).
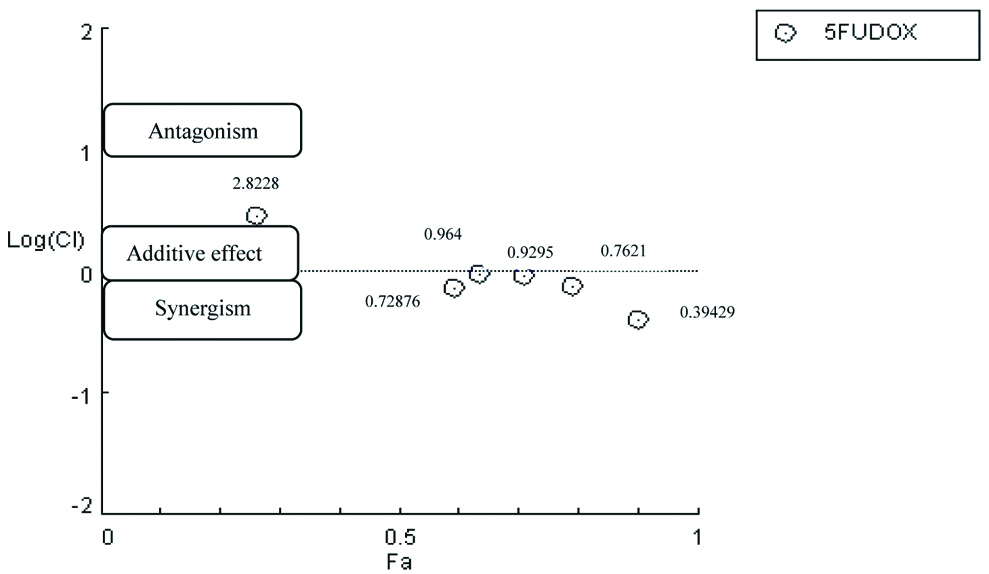
Dose-dependent response curves represent a qualitative graphic illustration for the activity of combined drug, while CI present a quantitative measure. The r values for all the experiments were 0.94 and higher, values of r were 0.95 or above indicating good conformity of the dose-dependent effect data with respect to the median-effect principle for in-vitro studies [22]. The CI value of the combination at 0.1 nMof DOX was 2.82283 that indicates slight antagonistic action. The CI value of 0.2 nM of DOX and 0.8 nM were 0.72876 and 0.76215 respectively that indicates moderate synergistic action, while at 0.4 nM the CI was 0.96471 indicating slight additive action. At 1 nM, the CI for the combined drug was 0.39429 that indicates strong synergistic action. These results suggests that the CI value decreased, as the concentration of DOX combination increased.
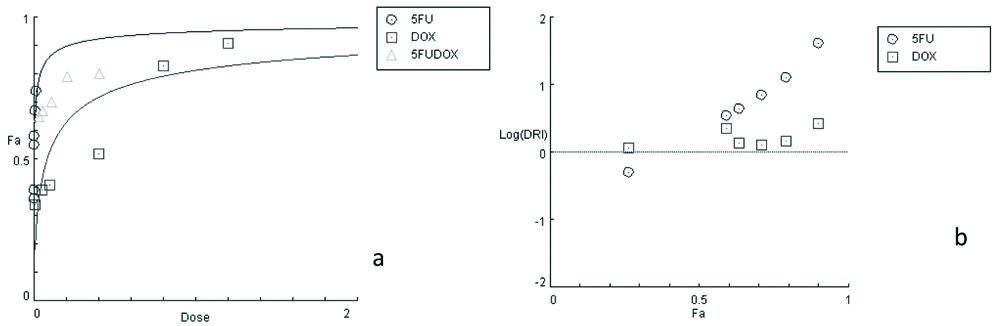
Dose-response curves illustrated in [Table/Fig-3] showed that DOX/5FU combination was significantly better than the single drug treatment confirming the favourable effect of 5FU in combination with DOX for treatment. The DRI values in [Table/Fig-4] show that the drug concentrations were decreased to reach the IC50, so, both drugs produced values with favourable DRI values ranging from (1.1 to 2.7).
a: Dose-response curves for single and combinations treatments of (DOX), (5FU) on MCF-7 cell line, b: Dose-reduction index DRI for 5FU and DOX.
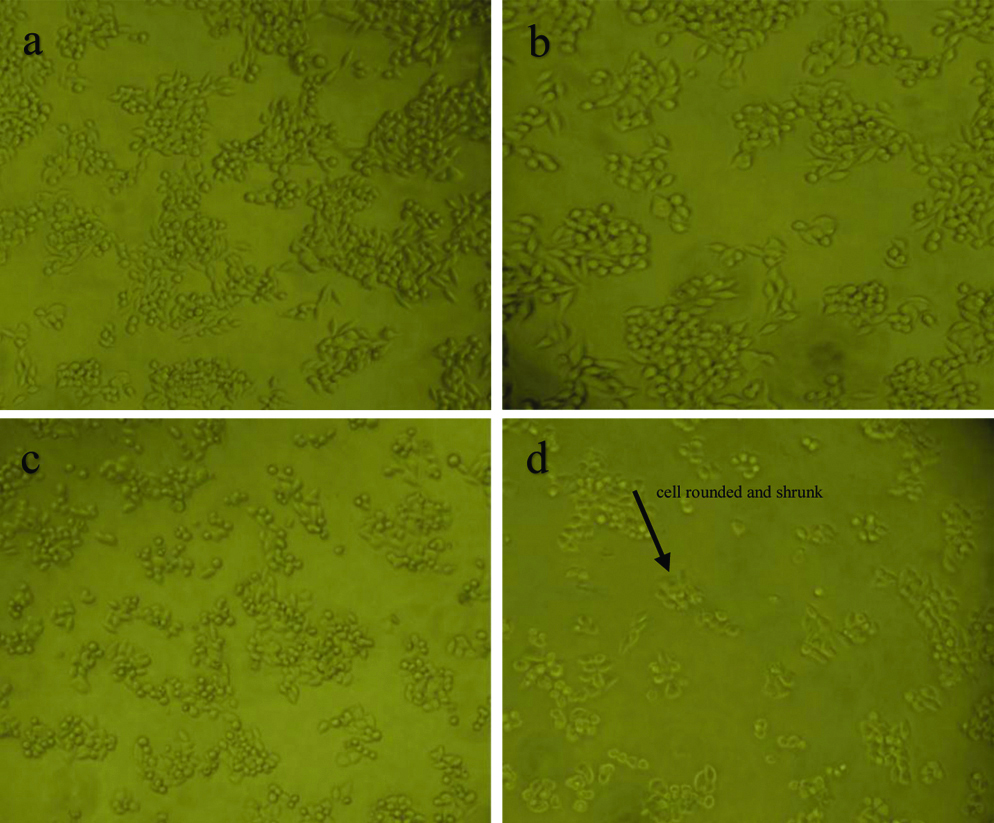
Results of microscopic observation of MCF-7 cells treated with DOX, 5FU, DOX+5FU illustrated in [Table/Fig-5]. The single treatment with DOX, 5FU of MCF-7 showed that the cells become rounded and detached from the surface of the dish, while the majority of the DOX/5FU treated cells were rounded, shrunk and many were detached, also apoptotic bodies was visible and the cell viability was decreased.
Morphological changes in MCF-7 cells by inverted microscope at (40X) after different treatment: (a) Untreated MCF-7 (control); (b) MCF-7 cells with DOX; (c) MCF-7 cells with 5FU; (d) MCF-7 cells DOX/5FU.
Discussion
This study revealed that the cytotoxicity of the combination of two drugs 5FU/DOX range from the addition to antagonism actions depending on the drug concentrations, according to combination index plot.
The quantitative approach such as CI, Fa, and DRI parameters are required for verifying synergy of drug combinations [23]. The effect of drug interaction is usually estimated by using the commonly applied median effect analysis [24,25]. Theoretically, combination therapy with therapeutic agents that are found to be efficient as mono-therapy in clinic should show good therapeutic effects without further toxicity at lower drug dosages [26]. One of the advantages of combination chemotherapy is improved patient compliance due to decrease in the dosage number [27]. The chemotherapeutic agents DOX and 5FU are used to treat cancer cells as they cause severe DNA damage, inducing cell death, they are also one of drugs in clinical medicine for which the qualitative spectrum of toxicity alters noticeably when the drug is used in different doses [28]. 5FU and DOX have been used in many clinical trials against breast carcinoma [29-31]. The present study showed that 5FU decreases the rates of cell viability when combined DOX against MCF-7 cell line. The CI analysis gives an uncomplicated demonstration of quantitative synergy or antagonism data. However, after being combined with 5FU, the DOX exhibited effective MCF-7 inhibition action especially at high concentrations.
Limitation
To improve more understanding on the mechanisms by which DOX and 5FU drugs work together to inhibit proliferation of cancer cells, pathways including flow cytometry, chromatin immunoprecipitation, assessment of therapeutic response in-vivo and angiogenesis needed to be studied.
Conclusion
The in-vitro preclinical study of DOX/5-FU combination at different low doses has successfully showed to improve the therapeutic index of both anti-cancer drugs, by enhancing their anti-tumour activity and/or by minimising their toxicity.