Introduction
Patients with Type 2 Diabetes Mellitus (T2DM) are at an increased risk of Cardiovascular Disease (CVD) with >50% mortality risk. In cases where a resting Electrocardiograph (ECG) fails to detect the abnormal cardiac function, serum High-Sensitivity C-Reactive Protein (hsCRP) levels and Tread Mill Test (TMT) variables are prescribed independently for the CVD risk prediction. A possible link between TMT variables and underlying inflammation needs to be substantiated clinically.
Aim
Evaluation of correlation between serum hsCRP levels and TMT variables in patients with T2DM.
Materials and Methods
Over a period of three months, Thirty T2DM patients without clinical evidence of Coronary Artery Disease (CAD) were evaluated for complete haemogram, fasting and post prandial blood sugar, lipid profile, and serum hsCRP levels. Standard multistage maximal exercise test was conducted on a motorized treadmill according to Bruce protocol. Spearmen’s correlation coefficient was used for statistical analysis.
Results
T2DM patients with higher serum hsCRP levels had lower exercise tolerance (r=-0.067; p=0.0001) and serum hsCRP levels increased with the duration of T2DM (r=0.55; p=0.002). Serum hsCRP levels and Heart Rate Recovery (HRR) at the end of first (r=-0.57) and second (r=-0.67) minute were statistically significant and showed negative correlation.
Conclusion
The results suggested a possible role of inflammation in the stress test responses in patients with T2DM without overt heart disease. Incorporating both serum hsCRP levels and TMT for the assessment and evaluation of T2DM patients can improve the predictive risk for CVD.
Cardiac autonomic neuropathy, Chronic hyperglycaemia, Exercise tolerance, Heart rate recovery, Inflammation
Introduction
Diabetes Mellitus (DM) is defined as a group of metabolic diseases characterised by hyperglycaemia due to defects in insulin secretion, its action, or both as stated by American Diabetes Association. The chronic hyperglycaemia can lead to long-term damage, dysfunction, and failure of various organs, especially the kidneys, nerves, heart, and blood vessels [1]. In 2010, 50.8 million cases of diabetes were reported in India and this number is presumed to rise to 87.0 million by 2030 [2].
Asian Indians have a greater degree of insulin-resistance in comparison to Caucasians, hence, leading to an increase in the prevalence of T2DM. The prevalence is as high as 13% in some rural and urban areas as corroborated by a study sponsored by the Indian Council of Medical Research [3]. The patients with T2DM are at an increased risk of CVD, and mortality rate due to CAD estimates to >50% [4-6]. In DM, asymptomatic CAD is mainly attributed to the underlying complication of Cardiac Autonomic Neuropathy (CAN), which may present as sudden death, Myocardial Ischemia (MI), arrhythmias, silent MI, or heart failure [7,8].
Current evidence suggests that decrease in exercise tolerance, reduced responses in heart rate and Blood Pressure (BP) and changes in cardiac output during exercise are due to the autonomic dysfunction. Studies further substantiate the fact that the increase in the inflammatory cytokines observed in patients with T2DM correlate well with the disturbances in the sympathovagal balance observed in them [9]. Serum hsCRP has proved to be a clinically useful biomarker in improving the CVD risk prediction, is based on the same rationale that the systemic inflammation plays a vital role in the pathogenesis of atherosclerosis and CVD [10,11].
Clinical and observational studies have reported that exercise capacity is a powerful predictor of cardiovascular as well as overall mortality [12,13]. It justifies the use of cardiac stress test as a non-invasive technique in detecting the subjects with increased CAD risk. This is also true of some of the variables of physical exercise, such as HRR, chronotropic response and DTS [14].
Serum hsCRP levels and exercise capacity by TMT are currently used to improve the CVD risk prediction in patients with T2DM. hsCRP levels in the serum can be readily measured by the method of nephelometry using a semi-autoanalyser but the need to rule out overt systemic inflammatory or immunologic disorders cannot be overstated as they can increase the levels of hsCRP [15]. Autonomic dysfunction and neuropathy, which are reflected, in part, by HRR after stress test can be used as potential risk markers of CVD mortality. Clinicians can advocate risk stratification in their practice by using this data. However, there is dearth of data regarding the association between the parameters of TMT and the inflammatory status, and whether it aids in better CVD risk prediction in T2DM, if used in conjunction. Further, there is paucity of Indian data regarding correlation between serum hsCRP levels, exercise capacity, and T2DM. Therefore, this study is an attempt to evaluate the correlation between serum hsCRP levels and parameters of TMT (total exercise capacity in seconds, HRR at the end of first and second minute) in patients with T2DM.
Materials and Methods
The present cross-sectional study was conducted at Shri BM Patil Medical College and Hospital, Karnataka during the period of July 2016 to September 2016.
Consecutive T2DM patients (diagnosed according to the WHO criteria) in the age group of 35-70 years, visiting the Diabetic Clinic at Medicine OPD of Shri BM Patil Hospital, Vijayapura were enrolled in the study over the period of three months [16]. T2DM patients on treatment with oral hypoglycemic agents and/or insulin, for at least six months, were selected. Patients presenting with signs and/or symptoms of overt CAD and those with history of treatment for coronary heart disease, congestive cardiac failure, MI, hypertension, angina pectoris, renal disease, thyroid disorder, ketosis or stroke were excluded from the study. Before the commencement of study, ethical clearance was obtained from the Institutional Ethical Committee (IEC ref no. 01/2016/dt/10/02/16). After explaining the aim and procedure of the study, a written informed consent was obtained from all the patients before data collection.
Data Collection
The patient’s personal history and occupational details were recorded using predesigned and pretested proforma. Data of participants, including name, age, sex, height and weight (for calculation of BMI) were collected. A meticulous systemic examination was conducted in all patient to rule out T2DM-induced systemic complications. After enrolling in the study, routine haemogram, fasting, post prandial blood sugar and lipid profile were measured. The participants were also subjected to TMT and assayed for serum hsCRP levels.
Procedure
Technique of treadmill test: The participants were instructed to wear comfortable shoes and loose-fitting clothes and to abstain from caffeine-containing beverages about three hours prior to stress test. A standard 12-lead ECG was performed followed by a torso ECG, which was obtained in the supine position and either in the sitting or standing position. BP was recorded in both the positions and the patients were instructed on how to perform the test.
Standard multistage maximal exercise test was performed on a motorized treadmill in line with the Bruce protocol [17]. The heart rate, BP, ECG and symptoms were recorded during first and second minute of exercise, at maximum exercise, and during recovery phase in the standing position and for each minute for at least 5-10 minutes in the recovery phase. HRR was defined as the maximum heart rate minus heart rate at specified time period after exercise, which represented the drop in the heart rate during that time interval. Predicted peak heart rate was calculated as 220 beats/min; maximum heart rate achieved during exercise and during the recovery phase was noted for each participant. The exercise stress test was terminated in cases of physical exhaustion or when maximum heart rate was greater than the age-predicted maximum heart rate. A computerised database was used to perform, analyse and reported according to a standard protocol.
Estimation of hsCRP Levels
The serum hsCRP levels were estimated by nephelometry method using a semi-autoanalyser (AGAPPE, Mispa UNO, Ref. no. 21103100) and commercially available kits from BN Systems, Dade Behring, Marburg, Germany [18]. The procedure had a sensitivity of 0.2 mg/L, and serum hsCRP levels <1 mg/L indicating low risk; 1-3 mg/L as normal risk and levels >3 mg/L as high risk according to the manufacturer’s instructions.
Statistical Analysis
The data were represented as mean±SD. Study variables were assessed for correlation by computing the correlation coefficients. The asymmetric distribution of serum CRP levels data, the natural logarithm of CRP transform was used as the dependent variable. Further, a stepwise log regression model was used for the multivariate analysis of CRP levels using Statistical Package for the Social Sciences (SPSS) version 17 and p-value <0.001 was considered to be statistically significant.
Results
Out of the 30 patients, 21 were male and 9 were female among them eighteen (60%) participants had ≤5 years of duration of T2DM followed by seven patients (23%) with a duration of 6-10 years, three patients (10%) with duration of 11-15 years, and two patients (7%) with a duration of 16-20 years. The mean hsCRP level (mg/dL) among males and females were 2.52±0.384 and 2.66±0.33, respectively [Table/Fig-1].
Baseline characteristics of the participants shown as mean±SD.
| Variables | Mean±SD |
|---|
| Age (years) | 54.4±5.65 |
| Body mass index (kg/m2) | 26.5±1.48 |
| Total cholesterol (mg/dL) | 280±14.14 |
| HDL-Cholesterol (mg/dL) | 49±15.5 |
| Duration of T2DM (years) | 8.5±2.12 |
| Fasting blood glucose (mg/dL) | 142±4.8 |
| hsCRP (mg/L) | 2.56±0.36 |
| Exercise time (min) | 7.25±1.78 |
| HRR at end of 1st minute (beats/min) | 16.66±6.046 |
| HRR at end of 2nd minute (beats/min) | 33.86±12.69 |
HDL: High density lipoprotein; T2DM: Type 2 diabetes mellitus; hsCRP: High-sensitivity C-Reactive protein; HRR: Heart rate recovery
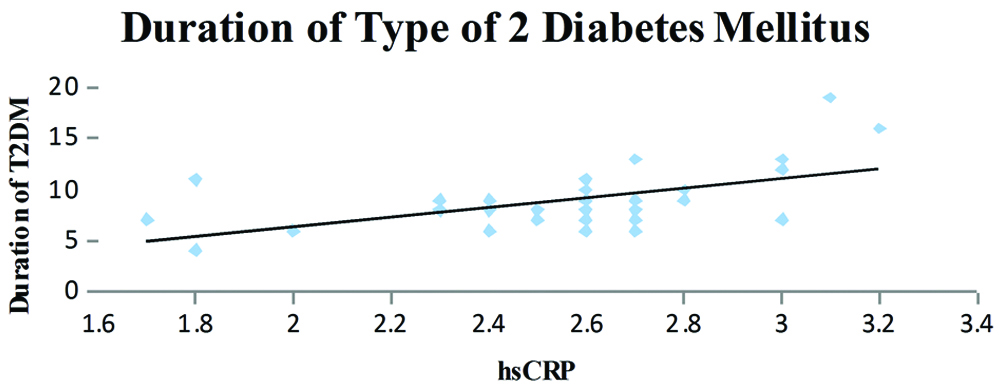
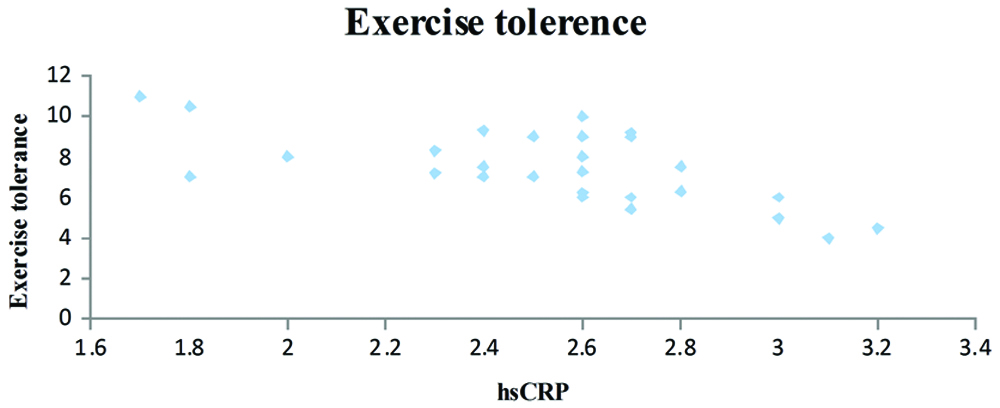
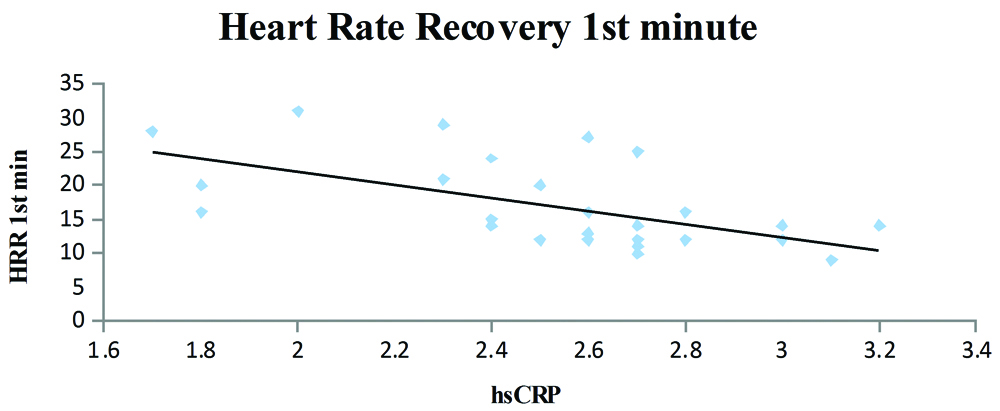
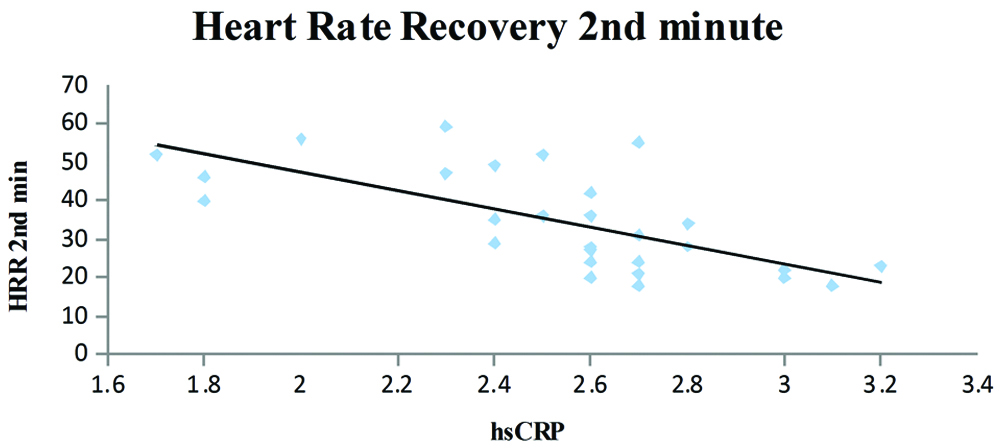
The T2DM patients with high serum hsCRP levels had low exercise tolerance when compared to patients with low serum hsCRP levels (r=-0.067 and p=0.0001) [Table/Fig-2]. A negative correlation was also observed between the serum hsCRP levels and HRR at the end of first (r=-0.57) and second minute (r=-0.67) and was statistically significant. Further, the serum hsCRP levels increased with the duration of T2DM (r=0.55; p=0.002) and the serum hsCRP levels were higher in patients with a poor diabetic control (FBS >126 mg/dL, PPBS >200 mg/dL).
Relationship between the logarithm of the hsCRP concentration and the studied variables.
| Variables | Coefficient | p-value |
|---|
| Age (years) | +0.0677 | 0.6365 |
| Body mass index (kg/m2) | +0.0008 | 0.88 |
| Exercise tolerance | -0.452 | 0.0001** |
| HRR at 1st minute (beats/min) | -0.3385 | 0.001* |
| HRR at 2nd minute (beats/min) | -0.4671 | 0.0001** |
| Duration of T2DM (years) | +0.3031 | 0.002* |
**Statistically highly significant; *Statistically significant; HRR: Heart rate recovery; T2DM: Type 2 diabetes mellitus; HRR: Heart rate recovery; T2DM: Type 2 diabetes mellitus
A statistically significant correlation was observed, the hsCRP levels increased in patients with longer duration of the T2DM [Table/Fig-3]. Patients with higher hsCRP levels had lower exercise capacity and the correlation was statistically significant [Table/Fig-4]. The HRR at the end of first minute was slower in patients who had higher hsCRP levels and was significant statistically [Table/Fig-5]. It was also observed that the patients who had higher hsCRP levels showed slower HRR at the end of second minute was significant statistically [Table/Fig-6].
Dispersion graphic of the high-sensitivity C-reactive protein concentration and Duration of Type 2 Diabetes Mellitus; (y=4.6913x - 2.9764; R2=0.3031; r=0.55, p=0.002).
Dispersion graphic of the high-sensitivity C-reactive protein concentration and exercise tolerance (y=-3.3011x+15.704; R2=0.452; r=-0.67; p=0.0001).
Dispersion graphic of the high-sensitivity C-reactive protein concentration and heart rate recovery at the end of first minute; y=-9.6913x+41.476; R2=0.3385; r=-0.57; p=0.001.
Dispersion graphic of the high-sensitivity C-reactive protein concentration and heart rate recovery at the end of second minute; y=-23.902x+95.055; R2=0.4671; r=-0.67; p=0.0001.
Discussion
The present study is among few studies which was conducted in Indian population to evaluate correlation between the serum hsCRP levels and TMT variables such as total exercise time and HRR at end of 1st minute and end of 2nd minute in T2DM patients without overt CAD [15].
In the present study, although serum levels of hsCRP and age were positively correlated, they were statistically insignificant. This can be attributed to the small sample size (n=30) of the study. Statistically significant (p=0.362) gender difference was not observed in the serum levels of hsCRP, this could be attributed to the fact that the male and female did not have equal representation in the sample recruited, which stands as one of the limitations of this study. Serum levels of the hsCRP were higher in patients with increased BMI levels. However, the correlation was statistically insignificant. On the contrary, some studies reported significant positive association between serum hsCRP levels and BMI [19]. The serum hsCRP levels are more correlated with abdominal obesity as reflected by higher waist circumference than BMI [20].
Although, BMI remains as one of the most commonly employed tool for the measurement of obesity in epidemiological studies, it cannot directly distinguish between the central and peripheral adiposity [21]. The exercise tolerance of patients with high serum hsCRP levels was poorer when compared to patients with low serum hsCRP levels (r= -0.67). These findings suggest that the inflammatory load is reflected on treadmill exercise test responses even in individuals without overt heart disease; moreover, the decrease in exercise capacity was in line with the underlying inflammation. Similarly, De Lemos ET et al., corroborated that decreased physical activity may lower the daily energy expenditure resulting in increased visceral fat leading to further activating the oxidative stress and inflammatory cascade [22]. Baseline hsCRP is negatively correlated with daily life activity level, indicating that regular exercise reduces general inflammatory status [23].
Brown and colleagues reported that even a single bout of exercise in untrained individuals can increase the activity of both circulating IL-6 and neutrophil counts and can have a range of health benefits [24]. Redox balance and metabolic status may also be physiologically involved as adaptive mechanisms to physical stress [25]. Regular exercise is associated with a decrease in the levels of inflammatory markers along with an increase in the levels of anti-inflammatory substances, thus corroborating the fact that exercise is anti-inflammatory in nature [26,27]. Therefore, regular and moderate exercise trainings should be advocated in the management of T2DM due to its established systemic protective effects [28]. In the present study, recovery was faster in patients with low serum hsCRP levels than in those with higher inflammatory load as evidenced by increased serum hsCRP levels (r=-0.57 and r=-0.67 for HRR at the end of first and the second minute, respectively). HRR is a non-invasive assessment of autonomic dysfunction and is known to be associated with adverse cardiovascular events and mortality. The differences in the HRR for individuals are largely due to the genetic differences, hence both the resting as well as post-exercise values should be considered when evaluating differences in autonomic nervous system control and predicting cardiac and all-cause mortality [29].
Various studies substantiate the fact that inflammatory cytokines can be modulated by the vagal stimulation, which may act through the cholinergic anti-inflammatory pathway [30,31]. These observations reflect on the possibility that slow HRR may be related to the underlying inflammation. Autonomic nervous system plays an important role in modulating HRR after TMT, and the slow heart rate recovery after the TMT can be a possible sign of CAN, which is often associated with MI of silent nature. Slow HRR, which is usually observed during the first 30 seconds of recovery after exercise could be due to reactivation of the parasympathetic and a decline in the sympathetic drive [32]. Hence, during interpretation of the routine exercise test, HRR after the exercise testing is of great importance.
The possible explanation may be that the abnormalities associated with the parasympathetic activation could be the probable pathophysiological link leading to the observation of a positive association between abnormal HRR after exercise stress test and increased mortality observed during the follow-up period [33]. Therefore, there is ample evidence to the fact that the abnormalities of the sympathovagal balance observed in patients with T2DM correlate with the activation of inflammatory cytokines. The present study suggests the combined use of serum hsCRP levels and various parameters of exercise stress test, such as exercise tolerance and HRR at the end of first and second minute for better risk stratification of CVD in T2DM patients. These parameters are positively correlated and appear to share a cause-effect relationship to a certain extent. This study further reinforces that exercise can be an effective adjunct therapy in the management of T2DM and the systemic complications, such as CVD.
Further, an important observation was that the serum hsCRP levels were inversely proportional to the exercise tolerance among the sample selected. The slow HRR at the end of first and second minute may reflect the early signs of autonomic dysfunction. These findings could possibly be due to the underlying inflammation and hold the potential of an independent marker of morbidity and mortality. Moreover, the therapeutic lifestyle changes, such as improving the cardiac fitness, which has the potential to address the inflammatory load as well as the early autonomic disturbance, are a part of CVD prevention strategy in patients with T2DM.
Conclusion
Early screening of patients with T2DM for evidence of CAD may prevent catastrophic cardiac events. Reducing the levels of inflammatory markers by improving the exercise capacity may be an additional incentive to stimulate sedentary individuals and improve physical conditioning through regular exercise. HRR at the end of first and second minute are simple observations of TMT, which can be implicated by clinicians for improved diagnosis and identification of CVD complications.
Limitation
The present study has certain limitations, which include small sample size and the lack of uniformity in terms of duration and other fitness parameters.
HDL: High density lipoprotein; T2DM: Type 2 diabetes mellitus; hsCRP: High-sensitivity C-Reactive protein; HRR: Heart rate recovery
**Statistically highly significant; *Statistically significant; HRR: Heart rate recovery; T2DM: Type 2 diabetes mellitus; HRR: Heart rate recovery; T2DM: Type 2 diabetes mellitus
[1]. American Diabetes AssociationDiagnosis and classification of diabetes mellitusDiabetes Care 2014 37(Suppl 1):S81-S90.10.2337/dc14-S08124357215 [Google Scholar] [CrossRef] [PubMed]
[2]. Shaw JE, Sicree RA, Zimmet PZ, Global estimates of the prevalence of diabetes for 2010 and 2030Diabetes Res Clin Pract 2010 87:4-14.10.1016/j.diabres.2009.10.00719896746 [Google Scholar] [CrossRef] [PubMed]
[3]. Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, Prevalence of diabetes and prediabetes in 15 states of India: Results from the ICMR-INDIAB population-based cross-sectional studyLancet Diabetes Endocrinol 2017 5(8):585-96.10.1016/S2213-8587(17)30174-2 [Google Scholar] [CrossRef]
[4]. Al-Nozha MM, Ismail HM, Al Nozha OM, Coronary artery disease and diabetes mellitusJ Taibah Univ Med Sci 2016 11(4):330-38.10.1016/j.jtumed.2016.03.005 [Google Scholar] [CrossRef]
[5]. Dodani S, Sharma GK, Presence of coronary artery disease in diabetic and non diabetic South Asian immigrantsIndian Heart J 2018 70(1):50-55.10.1016/j.ihj.2017.07.00929455788 [Google Scholar] [CrossRef] [PubMed]
[6]. Srinivasan MP, Kamath PK, Bhat NM, Pai ND, Bhat RU, Shah TD, Severity of coronary artery disease in type 2 diabetes mellitus: Does the timing matter?Indian Heart J 2016 68(2):158-63.10.1016/j.ihj.2015.08.00427133324 [Google Scholar] [CrossRef] [PubMed]
[7]. Serhiyenko VA, Serhiyenko AA, Cardiac autonomic neuropathy: risk factors, diagnosis and treatmentWorld J Diabetes 2018 9(1):1-24.10.4239/wjd.v9.i1.129359025 [Google Scholar] [CrossRef] [PubMed]
[8]. Bissinger A, Cardiac Autonomic Neuropathy: why should cardiologists care about that?J Diabetes Res 2017 2017:537417610.1155/2017/537417629214181 [Google Scholar] [CrossRef] [PubMed]
[9]. Vinik AI, Erbas T, Casellini CM, Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular diseaseJ Diabetes Investig 2013 4(1):4-18.10.1111/jdi.1204223550085 [Google Scholar] [CrossRef] [PubMed]
[10]. Kamath DY, Xavier D, Sigamani A, Pais P, High sensitivity C-reactive protein (hsCRP) & cardiovascular disease: An Indian perspectiveIndian J Med Res 2015 142(3):261-68.10.4103/0971-5916.16658226458341 [Google Scholar] [CrossRef] [PubMed]
[11]. Pfützner A, Schöndorf T, Hanefeld M, Forst T, High-sensitivity C-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: effects of insulin-sensitizing treatment with pioglitazoneJ Diabetes Sci Technol 2010 4(3):706-16.10.1177/19322968100040032620513338 [Google Scholar] [CrossRef] [PubMed]
[12]. Leeper NJ, Myers J, Zhou M, Nead KT, Syed A, Kojima Y, Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial diseaseJ Vasc Surg 2013 57(3):728-33.10.1016/j.jvs.2012.07.05123044259 [Google Scholar] [CrossRef] [PubMed]
[13]. Kokkinos P, Sheriff H, Kheirbek R, Physical inactivity and mortality riskCardiol Res Pract 2011 2011:92494510.4061/2011/92494521318105 [Google Scholar] [CrossRef] [PubMed]
[14]. Hong SP, Park WY, Jun SW, Bae KR, Lee YS, Lee JB, Prognostic value of chronotropic response in exercise treadmill test after coronary revascularizationEur Heart J 2014 35:978-79. [Google Scholar]
[15]. Kamath DY, Xavier D, Sigamani A, Pais P, High sensitivity C-reactive protein (hsCRP) & cardiovascular disease: An Indian perspectiveIndian J Med Res 2015 142(3):261-68. [Google Scholar]
[16]. Luong MW, Stress testing: A contribution from Dr Robert A. Bruce, Father of exercise cardiologyBCMJ 2016 58(2):70-76. [Google Scholar]
[17]. WHO Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia https://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ [Google Scholar]
[18]. https://www.cdc.gov/NCHS/data/nhanes/nhanes_09_10/CRP_F_met.pdf [Google Scholar]
[19]. Huffman FG, Whisner S, Zarini GG, Nath S, Waist circumference and BMI in relation to serum high sensitivity C-reactive protein (hs-CRP) in Cuban Americans with and without type 2 diabetesInt J Environ Res Public Health 2010 7(3):842-52.10.3390/ijerph703084220617007 [Google Scholar] [CrossRef] [PubMed]
[20]. Lapice E, Maione S, Patti L, Cipriano P, Rivellese AA, Riccardi G, Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese peopleDiabetes Care 2009 32(9):1734-36.10.2337/dc09-017619587368 [Google Scholar] [CrossRef] [PubMed]
[21]. Torun E, Cakir E, Ozgüç F, Ozgen IT, The effect of obesity degree on childhood pulmonary function testsBalkan Med J 2014 31(3):235-38.10.5152/balkanmedj.2014.1310125337419 [Google Scholar] [CrossRef] [PubMed]
[22]. deLemos ET, Oliveira J, Pinheiro JP, Reis F, Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes mellitusOxid Med Cell Longev 2012 2012:741545doi:10.1155/2012/74154522928086 [Google Scholar] [CrossRef] [PubMed]
[23]. Mouridsen MR, Nielsen OW, Carlsen CM, Mattsson N, Ruwald MH, Binici Z, High-sensitivity C-reactive protein and exercise-induced changes in subjects suspected of coronary artery diseaseJ Inflamm Res 2014 7:45-55.10.2147/JIR.S5436024715762 [Google Scholar] [CrossRef] [PubMed]
[24]. Brown WM, Davison GW, McClean CM, Murphy MH, A systematic review of the acute effects of exercise on immune and inflammatory indices in untrained adultsJ Sports Med 2015 1(1):3510.1186/s40798-015-0032-x26512338 [Google Scholar] [CrossRef] [PubMed]
[25]. Accattato F, Greco M, Pullano SA, Carè I, Fiorillo AS, Pujia A, Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjectsPloS one 2017 12(6):e017890010.1371/journal.pone.017890028582461 [Google Scholar] [CrossRef] [PubMed]
[26]. Sallam N, Laher I, Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseasesOxid Med Cell Longev 2015 2016:723963910.1155/2016/723963926823952 [Google Scholar] [CrossRef] [PubMed]
[27]. Pedersen BK, Anti-inflammatory effects of exercise: role in diabetes and cardiovascular diseaseEur J Clin Invest 2017 47(8):600-11.10.1111/eci.1278128722106 [Google Scholar] [CrossRef] [PubMed]
[28]. Qiu S, Cai X, Sun Z, Li L, Zuegel M, Steinacker JM, Heart rate recovery and risk of cardiovascular events and all-cause mortality: a meta-analysis of prospective cohort studiesJ Am Heart Assoc 2017 6(5):e00550510.1161/JAHA.117.005505 [Google Scholar] [CrossRef]
[29]. Nederend I, Schutte NM, Bartels M, Ten Harkel AD, de Geus EJ, Heritability of heart rate recovery and vagal rebound after exerciseEur J Appl Physiol 2016 116(11-12):2167-76.10.1007/s00421-016-3459-y27614881 [Google Scholar] [CrossRef] [PubMed]
[30]. Bonaz B, Sinniger V, Pellissier S, Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulationJ Physiol 2016 594(20):5781-90.10.1113/JP27153927059884 [Google Scholar] [CrossRef] [PubMed]
[31]. Nijhuis LE, Olivier BJ, Dhawan S, Hilbers FW, Boon L, Wolkers MC, Adrenergic β2 receptor activation stimulates anti-inflammatory properties of dendritic cells in vitroPloS one 2014 9(1):e8508610.1371/journal.pone.008508624465481 [Google Scholar] [CrossRef] [PubMed]
[32]. Hage FG, Iskandrian AE, Cardiovascular imaging in diabetes mellitusJ Nucl Cardiol 2011 18:959-65.10.1007/s12350-011-9431-721785921 [Google Scholar] [CrossRef] [PubMed]
[33]. Ghaffari S, Kazemi B, Aliakbarzadeh P, Abnormal heart rate recovery after exercise predicts coronary artery disease severityCardiol J 2011 18(1):47-54. [Google Scholar]