Introduction
Irritable Bowel Syndrome (IBS) is a functional gastrointestinal disorder which has a complex pathophysiology including the role of inflammation being recently studied.
Aim
To assess the role of mast cells and Intraepithelial Lymphocytes (IELs) in IBS patients by morphometry.
Materials and Methods
In this study, 148 cases of IBS using the Rome III criteria and 28 controls were taken. IBS patients were divided into two subgroups of Post Infectious (PI) and Non Post Infectious (NPI) subgroups on the basis of past history of episodes of gastroenteritis. All of them underwent full length colonoscopy and biopsy from the descending colon. Histopathology of these two were compared with special attention to morphometric count of IELs by “pin hole” method and mast cells using toluidine blue highlighting.
Results
Biopsies were unremarkable in 23(15.54%) patients, whereas 125 (84.46%) patients were diagnosed as Non Specific Colitis (NSC). The difference in IEL counts and mast cell counts were extremely significant (p<0.0001) in controls vs patients, controls vs PI-IBS patients and PI-IBS vs NPI-IBS subgroup whereas the difference was significant (p<0.001) in controls vs. NPI-IBS subgroup.
Conclusion
The present study concluded that mast cells and IELs show a significant difference between IBS and non IBS cases, and could have an important role to play in the pathophysiology of IBS.
Colitis, Gastroenteritis, Intraepithelial lymphocytes, Mast cells
Introduction
IBS is one of the common conditions encountered in gastroenterology practice, but ironically also the least well understood. It is a functional bowel disorder characterised by abdominal pain, cramps, changes in bowel habit, gassiness, bloating, and nausea, but without any specific diagnostic markers. Thus, the diagnosis is based on clinical presentation for which several symptoms based diagnostic criteria have been suggested time to time over the last two decades [1]. These include Manning, Rome I, Rome II , Rome III and most recently Rome IV criteria. The Rome criteria is the most widely accepted worldwide because of its reasonable sensitivity and specificity [2].
IBS presents clinically as one of these three predominant subtypes- (1) IBS with constipation (IBS-C); (2) IBS with diarrhoea (IBS-D) and (3) Mixed IBS (IBS-M).
According to onset of symptoms, IBS patients are further divided into Post Infectious IBS (PI-IBS) and Non Postinfectious IBS (NPI-IBS). PI-IBS is defined as Rome criteria positive IBS of acute onset developing after an infectious aetiology [3].
Lack of disease defining biomarkers makes the aetiology of this disease complicated with various factors like altered gastrointestinal motility, visceral hypersensitivity, post infectious reactivity, brain-gut interactions, alteration in faecal micro flora, bacterial overgrowth, barrier dysfunctions, food sensitivity, carbohydrate malabsorption, and intestinal inflammation being implicated in its pathogenesis. A genetic contribution also seems likely based upon the high degree of concordance seen among monozygotic twins as well as familial aggregations observed [4].
Several diseases can clinically mimic IBS, especially Coeliac disease and Inflammatory bowel diseases with IBS-D and colorectal cancer with IBS-C [5]. Testing for IgA Tissue transglutaminase, faecal calprotectin and tumor markers respectively may be helpful.
Histopathological examination along with techniques like special stains, immunohistochemistry and ultrastructure analysis reveal morphologic changes involving mast cells, lymphocytes, enterochromaffin cells and antigen presenting cells [6]. Additionally, mast cells have also been observed to be in closer proximity to colonic nerve endings and is said to correlate strongly with severity and frequency of pain and discomfort [7]. A significant correlation with mast cells densities with anxiety, depression and somatisation has also been reported [8].
Understanding of the alterations in the neuroimmune axis is essential both for establishing the aetiology and for the development of new therapies. Hence, this study was taken up to assess the role of immunoactivation in IBS patients in this region by doing the morphometry counts of IELs and mast cells.
Materials and Methods
The study was conducted over a period of one year. The study design was “case-control” and the pattern was “cross-sectional”. It comprised of 148 patients who presented at the Gastroenterology Department and 28 controls were also included. Proper clearance from the ethical committee was taken for this research. Proper consent from the patients as well as the controls were taken in writing at the time of enrollment. The patients were selected on the basis of ROME III criteria for IBS. The controls were the apparently healthy and motivated attendants of the patients who willfully agreed to undergo this free of cost study to rule out any incidental colonic pathology of which they may be totally unaware of.
After proper counselling and informed consent, all details of patients and controls including full history, abdominal ultrasound and clinical examination were noted. Thorough full length colonoscopy and guided biopsies were taken from descending colon in cases/controls where colonoscopy was normal. The biopsies were fixed in 10% formalin and processed routinely. The slides were stained with Haematoxylin and Eosin stain for histopathological examination and Toluidine Blue for highlighting mast cells. After screening the slide at low power, 10 fields that showed maximum density for the inflammatory cells (hotspots), were chosen in which counting of IELs’ was done by the “pinhole method” and given as the number per 100 epithelial cells and for the mast cells the average number of the counts obtained in each of the 10 fields was given as the number/hpf.
The cases were assigned into PI-IBS and NPI-IBS category based on the history of previous episodes of gastrointestinal infection. Amongst PI-IBS cases, a time interval of four weeks between the onset of past infectious episode and current symptoms pointing towards IBS was taken as a safe margin to avoid false interpretations of histopathological findings [9].
Results
The study comprised of 148 patients and 28 controls after excluding patients with abnormal colonoscopic findings/patients with histopathological findings consistent with diseases other than IBS. For all the control as well as the cases, the observations were carried out by two pathologists who were blinded for the clinical status of the slides seen. The interobserver variation was insignificant. The final counts for each of the controls and cases was taken as the mean of the counts reported by both the observers.
The mean age of presentation was 40 years (14.7) for controls with the M:F ratio being 3:1 whereas for cases it was 36.4 years (13.7) with the M:F ratio of 4:1. The most common presentation in cases was found to be abdominal pain (71.68%) which was defined as abdominal discomfort relieved by defaecation, followed by abdominal distension (66.26%), feeling of incomplete evacuation (52.40%), diarrhoea (56.62%) and constipation (21.08%). 24.33% of patients were labelled as PI-IBS and rest 75.67% as NPI-IBS. On histopathology, the biopsies from controls were normal [Table/Fig-1] whereas amongst the cases, biopsies were unremarkable in 23(15.54%) patients, whereas in 125 (84.46%) patients, it was diagnosed as Non Specific Colitis (NSC) where sections showed the epithelial lining and crypts having raised IELs with edematous lamina propria showing mild to moderate infiltration by inflammatory infiltrate comprising mainly of mast cells, lymphocytes, and few eosinophils. [Table/Fig-2]. The IEL counts in the controls ranged from 1-2/100 EC and in IBS from 3-5/100 EC and was higher in PIIBS than NPIIBS cases [Table/Fig-3,4 and 5]. The mast cell counts in the controls ranged from 0-2/hpf and in IBS from 2-4/hpf and was higher in PIIBS than NPIIBS cases [Table/Fig-6,7 and 8].
Normal biopsy- Section showing mild mononuclear and eosoniphilic inflammation in surface and crypt lining as well as in lamina propria; H and E, 400X.
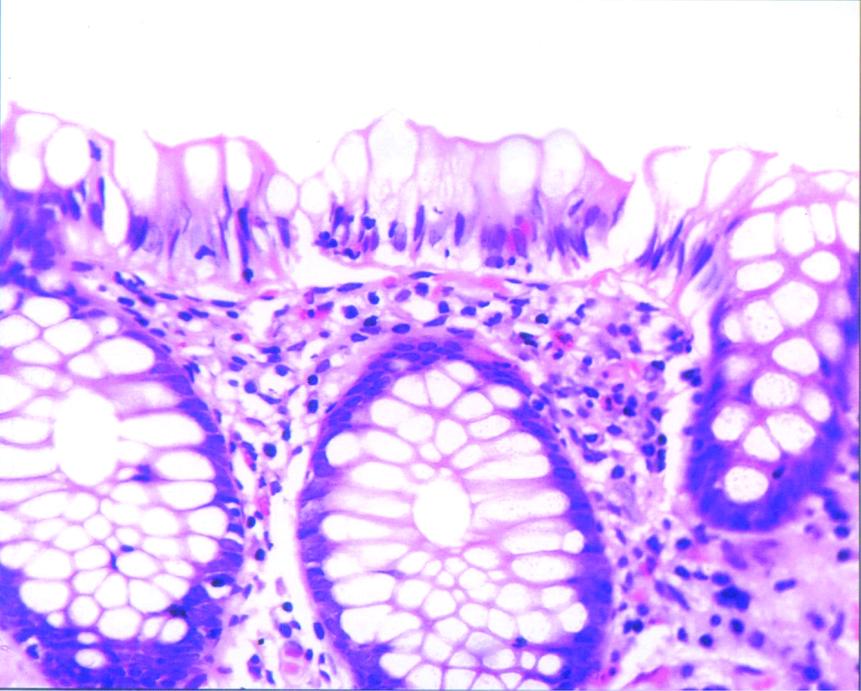
Non specific colitis- Section showing normal appearing crypts with increased mononuclear cells in the lining and lamina propria; H and E, 400X.
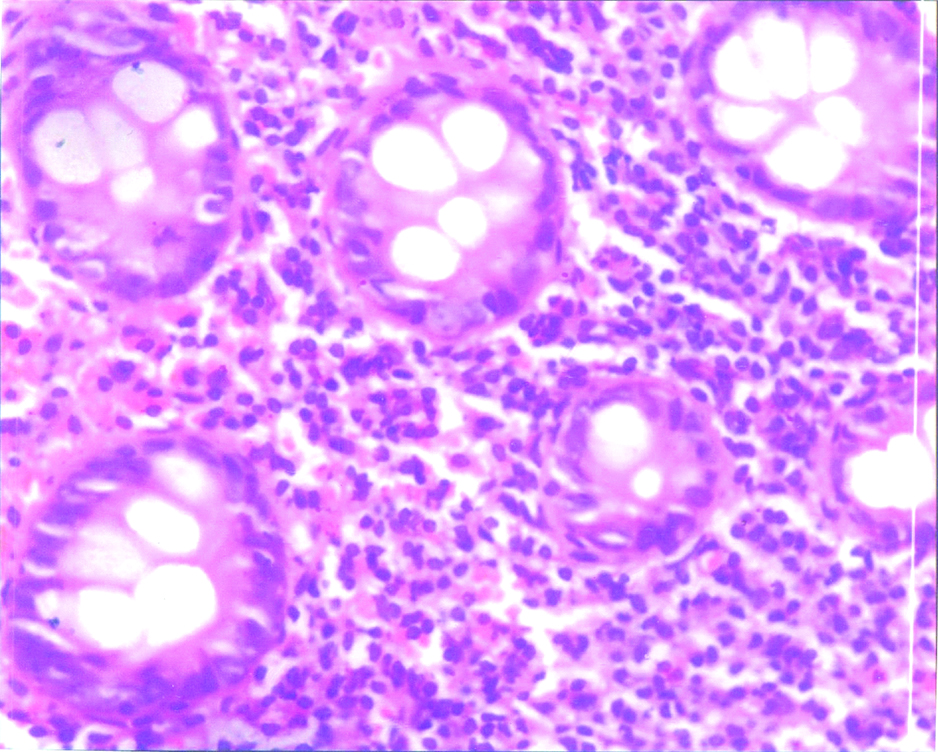
Intra epithelial lymphocytes in post infectious irritable bowel syndrome-section showing moderately increased intraepithelial lymphocytes in the crypt lining (↑); H and E, 400X.
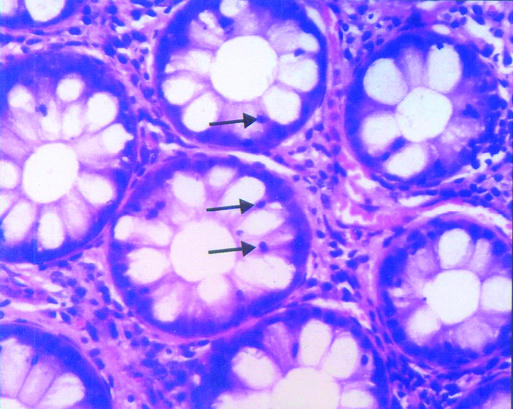
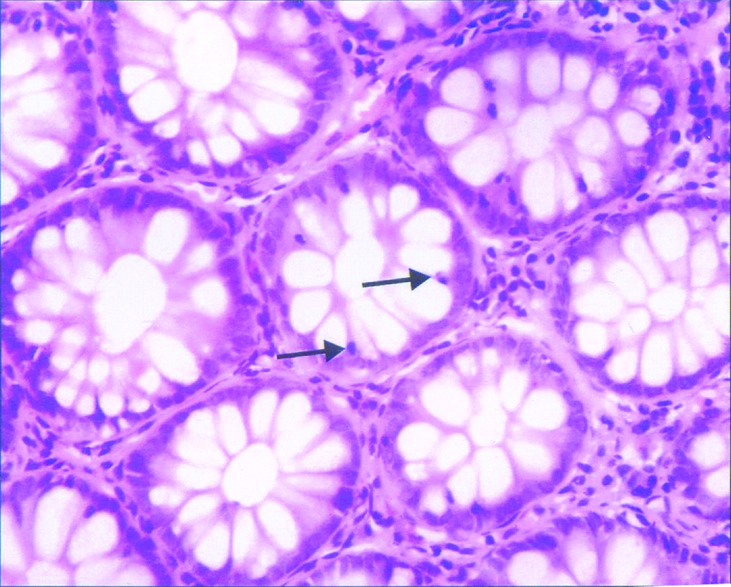
Intra epithelial lymphocytes in non post infectious irritable bowel syndrome-section showing mildly increased intraepithelial lymphocytes in the crypt lining (↑); H and E, 400X.
Comparison of intra epithelial lymphocyte counts in controls and irritable bowel syndrome patients.
| Control (A) | IBS (B)● | PIIBS (C)■ | NPIIBS◆ (D) | p-value |
|---|
| N=28 | N=148 | N=36 | N=112 | A v/s B | A v/s C | A v/s D | C v/s D |
| 1.32±0.74 | 3.22±0.76 | 4.51±1.4 | 3.64±0.8 | <0.0001 [ES]* | <0.0001 [ES]* | <0.0001 [ES]* | <0.005 [VS]# |
*Es=Extremely significant
#Vs=Very significant
●Irritable bowel syndrome; ■Post infectious irritable bowel syndrome; ◆Non post infectious irritable bowel syndrome
Mast cells in Post infectious irritable bowel syndrome-section showing moderately increased mast cells in the lamina propria (↑); Toluidine Blue, 400X.
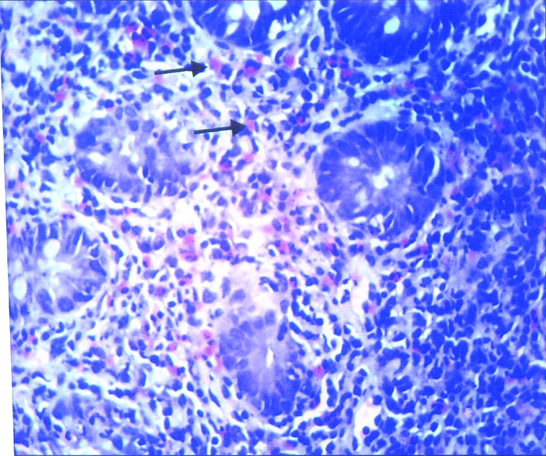
Mast cells in post infectious irritable bowel syndrome-section showing mildly increased mast cells in the lamina propria (↑); Toluidine Blue. 400X.
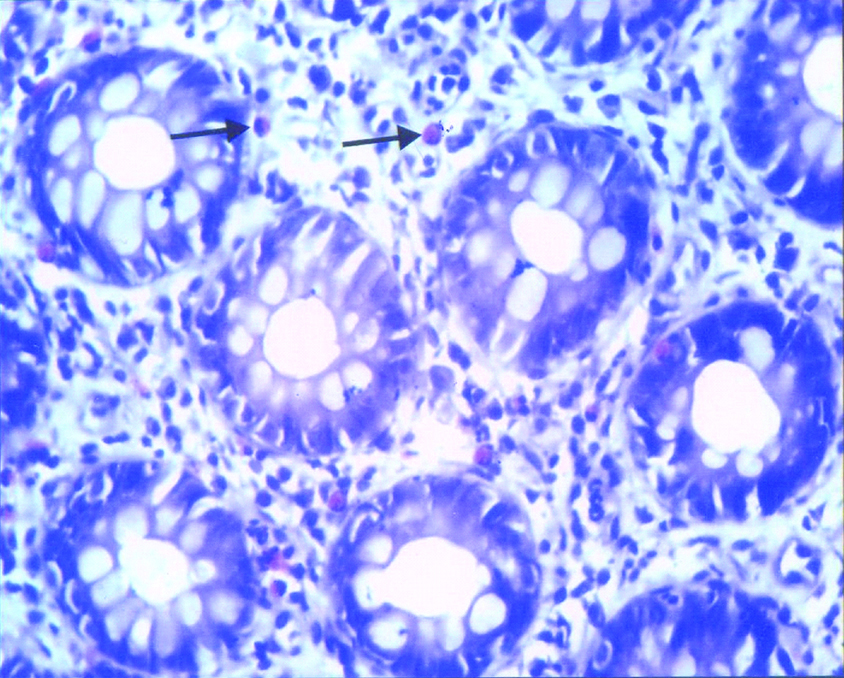
Comparison of mast cell counts in controls and irritable bowel syndrome patients.
| Control (A) | IBS (B)● | PIIBS (C)■ | NPIIBS (D)◆ | p-value |
|---|
| N=28 | N=148 | N=36 | N=112 | A v/s B | A v/s C | A v/s D | C v/s D |
| 1.29±0.8 | 2.88±1.26 | 3.08±0.9 | 2.63±0.64 | <0.0001 [ES]* | <0.0001 [ES]* | <0.0001 [ES]* | <0.0015 [VS]# |
*Es=Extremely significant; #Vs=Very significant
●Irritable bowel syndrome; ■Post infectious irritable bowel syndrome; ◆Non post infectious irritable bowel syndrome
Discussion
People with functional gastrointestinal disorders visiting healthcare centers account for up to one third of patient consultations [10]. The prevalence of IBS in Asia varies from 4 to 20% in different parts-in India this being from 4 to 8% [11]. The highest incidence have been reported in the second to fourth decades and more in women compared to men in studies from western countries [12]. In the present study also the affected cases ranged from 23 to 50 years. A significant male dominance (M:F=4:1) was seen which could be because in our society males are the major working population and hence more prone to work related stress.
In present study, biopsies were unremarkable in 23 (15.54%) patients, whereas in 125 (84.46%) patients, it was diagnosed as Non Specific Colitis (NSC) where sections showed low grade inflammation with raised IELs (3.22±0.68/100 cells) seen in the surface and crypt epithelium, and edematous lamina propria showing inflammatory infiltrate comprising mainly of mast cells (2.73±1.1 cells/hpf), lymphocytes and few eosinophils. Such findings have been reported in many other studies as well where compared to controls, IBS patients showed more chronic inflammation, but not severe enough to label it into any other definitive type of colitis [13].
“Microscopic Colitis”, where the lymphocytes are usually more than 20/100 cells [14] and “Mastocytic Enterocolitis” where mast cells are more than 20/hpf in the gastrointestinal epithelial lining [15] may clinically mimic IBS [16]. The recognition of these entities are essential because of the markedly different treatment protocol [17].
In our study, we found a significant increase in the IELs especially in the PI-IBS group. This was quite similar to the findings of various studies that conducted biopsies from different segments of intestine and reported T lymphocytes to be significantly increased in lamina propria, crypts and surface epithelium in IBS patients as compared to controls. Bashashati M et al., and Kim HS et al., reported the CD3+T cells were increased in the rectosigmoid and the descending colon of the PIIBS patients [18,19]. While the latter reported raised CD8+ IELs as well, the former did not find any significant change in their numbers. Martin-Viñas JJ et al., too had similar observations in their review article [20]. Sundin J et al., reported that the number of Lamina Propria Lymphocytes (LPLs) in PI-IBS was significantly increased compared to those in healthy controls and were both CD4(+) and CD8(+). However, the number of CD19(+) LPLs was decreased in PI-IBS patients compared to healthy controls [21].
However, few studies have contradictory observations. Studies by Chadwick VS et al., and Lee KJ et al., showed no change in the number of CD3+ and CD8+ cells in patients with IBS [22,23] Braak B et al., reported the CD8+ T lymphocyte counts to be lower in IBS patients than in healthy controls in descending colon biopsy specimen whereas the CD3+ T cells showed no difference [24].
As for the mast cells, they were found to be significantly increased in both PI-IBS as well as NPI-IBS patients in comparison to controls which was again quite similar to reports of many other studies [23] Braak B et al., reported the CD8+ T lymphocyte counts to be lower in IBS patients than in healthy controls in descending colon biopsy specimen whereas the CD3+ T cells showed no difference [24].
As for the mast cells, they were found to be significantly increased in both PI-IBS as well as NPI-IBS patients in comparison to controls which was again quite similar to reports of many other studies [8,19,23]. Bashashati M et al., Wouters MM et al., and Lazardis N et al., in their review on role of mast cells in IBS have mentioned many studies where their numbers were found to be increased in IBS patients in biopsies taken from various parts of the intestine, both small and large as well as rectum [18,25,26]. Though most of the studies mentioned used immunohistochemistry for detection, Wang X et al., reported their raised numbers in the duodenal biopsies using toluidine blue [27]. Dunlop SP et al., in contrast reported more numbers of mast cells in NPI-IBS group compared to PI-IBS group [28].
However, there are a few studies that have reported conflicting results with no change seen in the absolute number of mast cells in IBS [25,26,29,30]. Braak B et al., have even reported their decreased numbers in IBS patients compared to controls and have emphasised upon that it is rather the activation state of mast cells which gets altered in IBS patients and are responsible for their symptoms [24].
As put forth by Wouters MM et al., this high degree of inconsistency can be ascribed to numerous reasons like absence of standardisation on the methodology of counting, differences in categories of patient selection (PI IBS vs NPIIBS/IBS-C vs. IBS-D), ethnicity, location chosen for the biopsy (not all segments of the intestine may be truly representative of the degree of disease), along with many other uncontrolled potential confounding factors [25].
Limitation
The main limitation of the study were that the sample size was small and help of immunohistochemistry was not taken which could have further improved the study.
Conclusion
The present study showed that mast cells and IELs are significantly higher in IBS patients, especially in post infectious ones when compared to controls. Hence, it is very likely that they play an important role in the pathophysiology of IBS and may require a different treatment strategy.
Future Directions
Studies done in IBS patients in Indian subcontinent are limited, dealing mainly with the epidemiological part. To the best of knowledge and research, studies based on quantification of mast cells and IEL’s in IBS patients are negligible. Hence, future studies on larger cohorts with use of advanced techniques are recommended to enhance our understanding of this complex disease and better management of these patients.
*Es=Extremely significant
#Vs=Very significant
●Irritable bowel syndrome; ■Post infectious irritable bowel syndrome; ◆Non post infectious irritable bowel syndrome
*Es=Extremely significant; #Vs=Very significant
●Irritable bowel syndrome; ■Post infectious irritable bowel syndrome; ◆Non post infectious irritable bowel syndrome
[1]. Owyang C, Irritable bowel syndrome. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, editorsHarrisons Principles of Internal Medicine 2008 17th edNew YorkMcGraw Hill Publications:1899-1903. [Google Scholar]
[2]. Lacy BE, Patel NK, Rome Criteria and a Diagnostic Approach to Irritable Bowel SyndromeJ Clin Med 2017 6(11):9910.3390/jcm611009929072609 [Google Scholar] [CrossRef] [PubMed]
[3]. Vicario M, Gonzalez-Castro AM, Martinez C, Lobo B, Pigrau M, Guilarte M, Increased humoral immunity in the jejunum of diarrhoea predominant irritable bowel syndrome associated with clinical manifestationsGut 2015 64(9):1379-88.10.1136/gutjnl-2013-30623625209656 [Google Scholar] [CrossRef] [PubMed]
[4]. Saha L, Irritable bowel syndrome: Pathogenesis, diagnosis, treatment and evidence based medicineWorld J Gastroenterol 2014 20(22):6759-73.10.3748/wjg.v20.i22.675924944467 [Google Scholar] [CrossRef] [PubMed]
[5]. Chey WD, Kurlander J, Eswaran S, Irritable bowel syndrome: A clinical reviewJAMA 2015 313(9):949-58.10.1001/jama.2015.095425734736 [Google Scholar] [CrossRef] [PubMed]
[6]. Hughes PA, Zola H, Pentilla I A, Blackshow LA, Andrews J M, Krumbieged D, Immune activation in Irritable bowel syndrome: Can neuroimmune interactions explain symptoms?Am J Gastroenterol 2013 108(7):1066-74.10.1038/ajg.2013.12023649183 [Google Scholar] [CrossRef] [PubMed]
[7]. Park H, The pathophysiology of irritable bowel syndrome: inflammation and motor disorderKorean J Gastroenterol 2006 47(2):101-10. [Google Scholar]
[8]. Piche T, Saint-Paul MC, Dainese R, Marine-Barjoan E, Iannelli A, Montoya ML, Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndromeGut 2008 57(4):468-73.10.1136/gut.2007.12706818194987 [Google Scholar] [CrossRef] [PubMed]
[9]. Borgaonkar MR, Ford DC, Marshall JK, Churchill E, Collins SM, The incidence of irritable bowel syndrome among community subjects with previous acute enteric infectionDig Dis Sci 2006 51(5):1026-32.10.1007/s10620-006-9348-116758307 [Google Scholar] [CrossRef] [PubMed]
[10]. Okumara T, Tanno S, Ohhira M, Tanno S, Prevalence of functional dyspepsia in an out patient clinic with primary care physicians in JapanJ Gastroenterol 2010 45(2):187-94.10.1007/s00535-009-0168-x19997854 [Google Scholar] [CrossRef] [PubMed]
[11]. Ghoshal UC, Abraham P, Bhatt C, Choudhuri G, Bhatia SJ, Shenoy KT, Epidemiological and clinical profile of irritable bowel syndrome in India. Report of the Indian Society of Gastroenterology Task ForceIndian J Gastroenterol 2008 27(1):22-28. [Google Scholar]
[12]. Mayer EA, Naliboff B, Lee O, Munakata J, Chang L, Review article: Gender related differences in functional gastrointestinal disordersAliment Pharmacol. Ther 1999 13(2):65-69.10.1046/j.1365-2036.1999.00008.x10429743 [Google Scholar] [CrossRef] [PubMed]
[13]. Kirsch RH, Riddell R, Histopathological alterations in Irritable Bowel SyndromeMod Pathol 2006 19(2):1638-45.10.1038/modpathol.380070417013373 [Google Scholar] [CrossRef] [PubMed]
[14]. Ucmak F, Goral V, Firat U, Mete N, Rate of microscopic colitis and cytokine levels in patients with irritable bowel syndromeActa Medica Mediterranea 2015 31(1):103-08. [Google Scholar]
[15]. Ramsay DB, Stephen S, Borum M, Voltiaggio L, Doman DB, Mast cells in gastrointestinal diseaseGastroenterol Hepatol 2010 6(12):772-77. [Google Scholar]
[16]. Kamp EJCA, Kane JS, Ford AC, Irritable bowel syndrome and microscopic colitis: A systematic review and meta analysisClin Gastroenterol Hepatol 2016 14(5):659-68.10.1016/j.cgh.2015.09.03126453949 [Google Scholar] [CrossRef] [PubMed]
[17]. Misra V, Misra SP, Dwivedi M, Singh PA, Agarwal V, Microscopic colitis is patients presenting with chronic diarrhoeaIndian J Pathol. Microbiol 2010 53:15-19.10.4103/0377-4929.5917620090215 [Google Scholar] [CrossRef] [PubMed]
[18]. Bashashati M, Moossavi S, Cremon C, Barbaro MR, Moraveji S, Talmon G, Colonic immune cells in irritable bowel syndrome: A systematic review and meta-analysisNeurogastroenterol Motil 2018 30(1)doi: 10.1111/nmo.13192. Epub 2017 Aug 2910.1111/nmo.1319228851005 [Google Scholar] [CrossRef] [PubMed]
[19]. Kim HS, Lim JH, Park H, Lee SI, Increased immunoendocrine cells in intestinal mucosa of post infectious irritable bowel syndrome patients 3 years after acute Shigella infection- an observation in small case control studyYonsei Med J 2010 51(1):45-51.10.3349/ymj.2010.51.1.4520046513 [Google Scholar] [CrossRef] [PubMed]
[20]. Martin-Viñas JJ, Quigley EM, Immune response in irritable bowel syndrome: A systematic review of systemic and mucosal inflammatory mediatorsJ Dig Dis 2016 17(9):572-81.10.1111/1751-2980.1237927426409 [Google Scholar] [CrossRef] [PubMed]
[21]. Sundin J, Rangel I, Kumawat AK, Hultgren-Hörnquist E, Brummer RJ, Aberrant mucosal lymphocyte number and subsets in the colon of post-infectious irritable bowel syndrome patientsScand J Gastroenterol 2014 49(9):1068-75.10.3109/00365521.2014.92698224919810 [Google Scholar] [CrossRef] [PubMed]
[22]. Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Activation of the mucosal immune system in irritable bowel syndromeGastroenterology 2002 122(7):1778-83.10.1053/gast.2002.3357912055584 [Google Scholar] [CrossRef] [PubMed]
[23]. Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW, The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factorsJ Gastroenterol Hepatol 2008 23(11):1689-94.10.1111/j.1440-1746.2008.05574.x19120860 [Google Scholar] [CrossRef] [PubMed]
[24]. Braak B, Klooker TK, Wouters MM, Welting O, van der Loos CM, Stanisor OI, Mucosal immune cell numbers and visceral sensitivity in patient in irritable bowel syndrome: is there any relationship?Am J Gastroenterol 2012 107(5):715-26.10.1038/ajg.2012.5422488080 [Google Scholar] [CrossRef] [PubMed]
[25]. Wouters MM, Vicario M, Santos J, The role of mast cells in functional GI disordersGut 2016 65(1):155-68.10.1136/gutjnl-2015-30915126194403 [Google Scholar] [CrossRef] [PubMed]
[26]. Lazaridis N, Germanidis G, Current insights into the innate immune system dysfunction in irritable bowel syndromeAnn Gastroenterol 2018 31(2):171-87.10.20524/aog.2018.022929507464 [Google Scholar] [CrossRef] [PubMed]
[27]. Wang X, Li X, Ge W, Huang J, Li G, Cong Y, Quantitative evaluation of duodenal eosinophils and mast cells in adult patients with functional dyspepsiaAnn Diagn Pathol 2015 19(2):50-56.10.1016/j.anndiagpath.2015.02.00125735567 [Google Scholar] [CrossRef] [PubMed]
[28]. Dunlop SP, Jenkins D, Spiller RC, Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndromeAm J Gastroenterol 2003 98(7):1578-83.10.1111/j.1572-0241.2003.07542.x12873581 [Google Scholar] [CrossRef] [PubMed]
[29]. Sethi A, Jain P, Roland BC, Kinzel J, Gibson J, Schrader R, Performing colonic mast cell counts in patients with chronic diarrhea of unknown etiology has limited diagnostic useArch Pathol Lab Med 2015 139(2):225-32.10.5858/arpa.2013-0594-OA25611105 [Google Scholar] [CrossRef] [PubMed]
[30]. Doyle LA, Sepehr GJ, Hamilton MJ, Akin C, Castells MC, Hornick JL, A clinicopathologic study of 24 cases of systemic mastocytosis involving the gastrointestinal tract and assessment of mucosal mast cell density in irritable bowel syndrome and asymptomatic patientsAm J Surg Pathol 2014 38(6):832-43.10.1097/PAS.000000000000019024618605 [Google Scholar] [CrossRef] [PubMed]