Head and neck cancers are the most common cause of cancer related to mortality and morbidity in India [1]. According to GLOBOCAN 2012, worldwide incidence and mortality of head and neck cancers (lip, oral cavity, pharynx and larynx) are 4.2% and 4.0% respectively. In India approximately 1.5 lac new cases are detected every year, accounting for approximately 20% of all cancers diagnosed every year [1,2]. Approximately, 60%-80% are diagnosed with locally advanced disease and 54% have lymph node involvement at the time of presentation [3,4]. The prognosis of patients with LAHNSCC is poor and five year survival rate with conventional RT is 40-50% [5]. Addition of concurrent chemotherapy to the radiotherapy has improved survival rates by 6-8% [6]. Hence, the realisation that conventional fractionation may not be the best fractionation for all situations has led to the concept of altered fractionation in RT. Various altered fractionation schemes such as hyperfractionation and accelerated fractionations have been used in past two decades in order to increase loco-regional control and survival. Keeping in mind the radio-biological aspects of accelerated fractionation RT which gives beneficial results by addressing the accelerated repopulation, we conducted this study to compare outcomes with accelerated fractionation versus conventional fractionation.
Materials and Methods
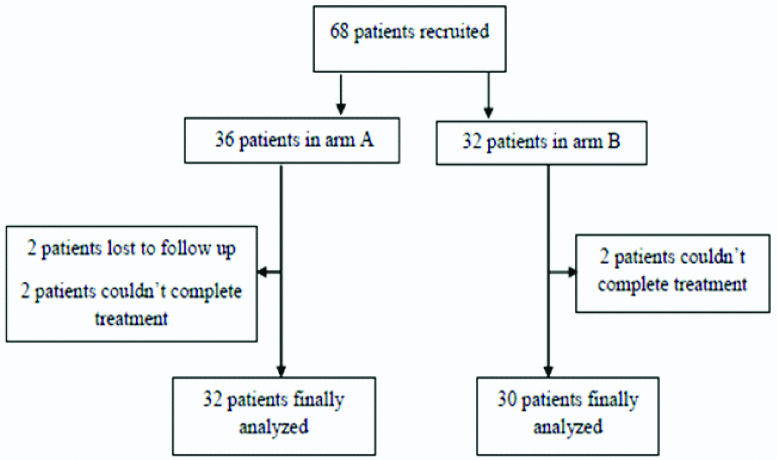
Patients of head and neck cancers presenting to the RT out-patient department, at the Sri Venkateswara Institute of Medical Sciences (SVIMS), Tirupati, during the period between March 2015 and March 2017 were included in this prospective randomised study after obtaining approval from Institutional Ethical Committee (IEC No. 444 dt.09.04.2015). A total of 68 patients were recruited into the study [Table/Fig-1]. These patients were randomly assigned to two arms, arm A (5 # per week) and arm B (6 # per week). Sixty two patients were included in final analysis (32 patients in arm A and 30 in arm B).
Flow chart showing the patient distribution in both arms.
Inclusion Criteria
- Histopathologically proven squamous cell carcinoma of head and neck (lip, oral cavity, oropharynx, hypopharynx and larynx).
- Patients with stage I to stage IVB according to American Joint Committee on Cancer (AJCC) staging manual, edition 7 [7].
- Eastern Co-operative Oncology Group (ECOG) performance status: 0-2.
- Patients who are willing to give approved informed consent.
Exclusion Criteria
- Patients previously treated with surgery or RT or induction chemotherapy for head and neck cancers.
- Patients with distant metastasis.
- Pregnant women and lactating mothers.
- Patients who are not willing to give informed consent.
Pretreatment evaluation:
- Complete history and physical examination.
- Biopsy
- Complete blood picture, renal function test and liver function test.
- Computed Tomography of head and neck (plain and contrast) to know local extent of tumour and for staging purpose.
Study Technique
Randomisation code was generated before starting the study. A total of 68 patients were taken into the study and randomly assigned to two arms A and B. Opaque sealed envelope method was used for treatment allocation. Patients assigned to arm A received radiation dose of 66Gy in 33 fractions at 2Gy per fraction one fraction a day five fractions per week for five consecutive days from Monday to Friday. Patients assigned to arm B received same total dose of 66Gy in 33 fractions at 2Gy per fraction six fractions per week one fraction a day for six consecutive days from Monday to Saturday. All the patients were treated either with 3-D Conformal Radiotherapy Technique (3-D CRT) or with Intensity Modulated Radiotherapy Technique (IMRT). Target volumes were delineated as per International Commission on Radiological Units and measurements (ICRU 50) [8]. Concurrent chemotherapy with weekly cisplatin 40 mg/m2 was given to the patients in both the arms who were medically fit for chemotherapy.
Measurement of Toxicities
Acute toxicities were assessed for skin, mucous membranes and dysphagia once weekly while on treatment and monthly for two months post-treatment and late toxicities were assessed for subcutaneous fibrosis, xerostomia and dysphagia after three months from the start of treatment till last follow-up and graded according to the Radiation Therapy Oncology Group (RTOG) morbidity scoring criteria [9]. The scores were based on the patient’s subjective symptoms, objective examination findings and treatment of the symptoms.
Response Evaluation
Response was assessed according to response evaluation criteria in solid tumours (RECIST) version 1.1 [10] after eight weeks of completion of treatment by clinical evaluation and imaging as appropriate.
Statistical Analysis
Patients were equally distributed between the two arms. The association between two categorical variables was evaluated by Chi-square test. Local control, survival, and complication rates were calculated by the Kaplan Meier method, and the differences between groups were compared by the log-rank test. The p-values estimated were for a two-tailed test, and the significance level was set at 5%. All the analysis was done using SPSS software version 21.
Results
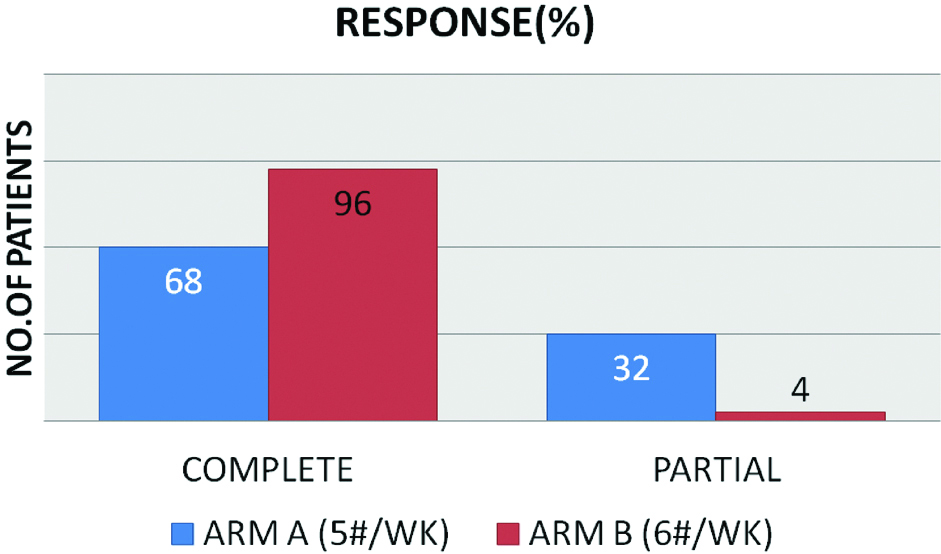
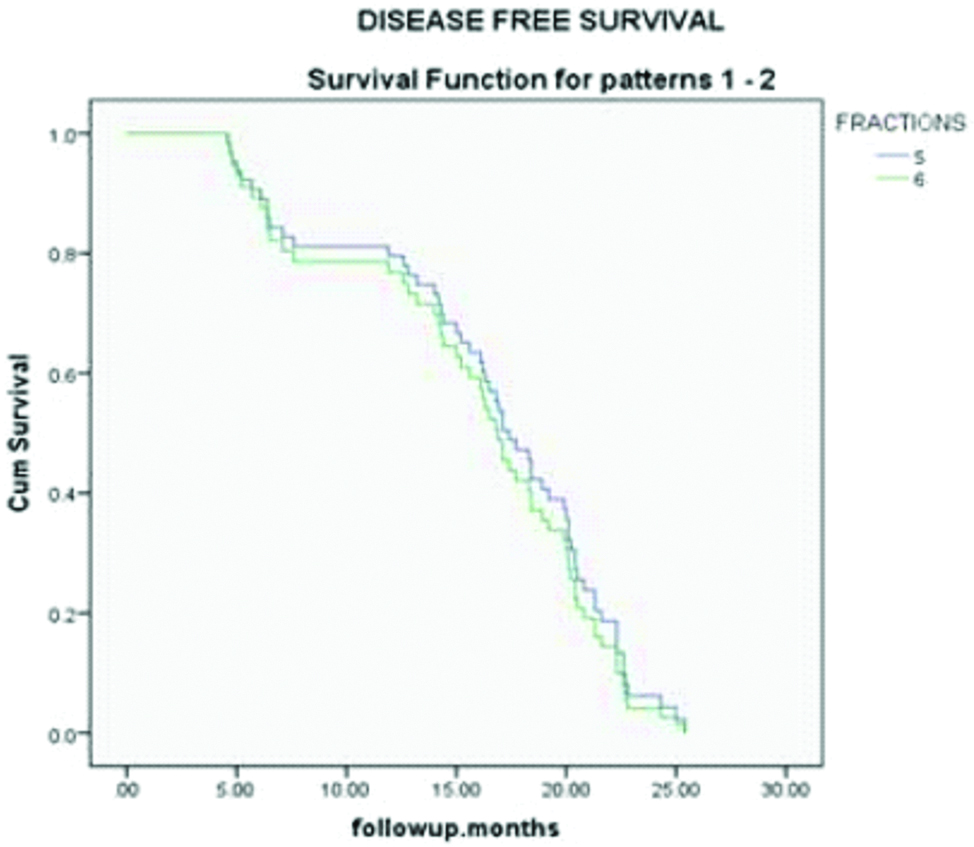
Baseline patient characteristics are shown in the [Table/Fig-2] and tumour characteristics are shown in the [Table/Fig-3]. There are 46 men and 16 women in this study with a median age of 54 years (30-66 years) at presentation. There were no significant differences in the baseline tumour characteristics between the two arms. Median follow-up period was 17 months (4.6-25.4 months). Most of the patients in both the arms (26 in arm A and 21 in arm B) received chemotherapy with weekly cisplatin 40 mg/m2 for 2-5 cycles. Out of 32 patients in arm A, after two months post RT, 22 patients achieved complete response and 10 patients had partial response and in arm B out of 30 patient, 29 patients achieved complete response and 1 patient had partial response. Most of the patients in arm B (6 #/week) had complete response which is statistically significant (p-value=0.003) [Table/Fig-4]. Most of the patients who had partial response have residual disease in neck nodes (7 patients in arm A and 0 in arm B). Two patients in arm A and one patient in arm B had residual primary. After a median follow-up of 17 months, disease free survival was calculated from the start of treatment till the occurrence of any event or last follow-up, with event being defined as loco-regional recurrence or distant metastasis. Three patients in arm A had loco-regional recurrence and one patient had distant metastasis to lungs. Three patients in arm B had loco-regional recurrence and none had distant metastasis. Disease free survival was slightly more in accelerated treatment arm compared to conventional treatment arm though not statistically significant (p-value 0.59) [Table/Fig-5].
Baseline patient characteristics.
| Arm A (5#/Week) N=32 | Arm B(6#/Week) N=30 |
|---|
| Age (years) |
| <40 | 4 | 5 |
| 41-50 | 9 | 9 |
| 51-60 | 14 | 13 |
| >60 | 5 | 3 |
| Sex |
| Male | 24 | 22 |
| Female | 8 | 8 |
| T stage | Arm A | Arm B |
|---|
| T2 | 5 | 6 |
| T3 | 10 | 4 |
| T4A | 17 | 18 |
| T4B | 0 | 2 |
| N stage |
| N0 | 5 | 6 |
| N1 | 5 | 8 |
| N2 | 18 | 16 |
| N3 | 4 | 0 |
| Stage group |
| Stage II | 1 | 3 |
| Stage III | 5 | 4 |
| Stage IVA | 19 | 21 |
| Stage IVB | 7 | 2 |
| Subsite |
| Oral cavity | 10 | 8 |
| Oropharynx | 8 | 6 |
| Hypopharynx | 10 | 12 |
| Supraglottis | 2 | 1 |
| Glottis | 2 | 3 |
Showing the response pattern in both arms.
Showing disease free survival in both arms.
Reactions
Acute radiation-induced morbidity [Table/Fig-6] was slightly more in the accelerated arm than in the conventional arm. More patients in accelerated arm experienced grade 2 and grade 3 skin reactions than in the conventional arm, but not statistically significant. Acute grade 2, grade 3 mucositis was also slightly more in accelerated arm which was not statistically significant. Additionally, mucositis persist longer in the patients who underwent accelerated treatment than in those who received conventional treatment. The more frequent mucosal reactions in the accelerated treatment group resulted in increased use of tube feeding during treatment compared with the conventional arm. Only one patient in conventional arm developed grade 4 mucositis which resulted in treatment delay of one week.
| Grade | Arm A (5#/Week) (%) | Arm B (6#/Week) (%) | p-value |
|---|
| Skin |
| 1 | 10 (31%) | 3 (10%) | 0.09 |
| 2 | 19 (59%) | 20 (66%) | 0.74 |
| 3 | 3 (0.9%) | 7 (23%) | 0.34 |
| Mucositis |
| 1 | 7 (21%) | 0 | 0.06 |
| 2 | 16 (50%) | 20 (66%) | 0.61 |
| 3 | 8 (25%) | 10 (33%) | 0.81 |
| 4 | 1 (3%) | 0 | 1 |
| Dysphagia |
| 1 | 2 (6%) | 1 (3%) | 1 |
| 2 | 19 (59%) | 13 (43%) | 0.37 |
| 3 | 11 (34%) | 16 (53%) | 0.44 |
Although late radiation induced morbidity [Table/Fig-7] in terms of grade 2 and grade 3 esophageal reactions were slightly more in accelerated arm in the form of tube dependency for feeding, there was no significant difference between the two treatment arms. Other late reactions like subcutaneous fibrosis and xerostomia were comparable in both the arms.
| Grade | Arm A (5#/Week) | Arm B (6#/Week) | p-value |
|---|
| Subcutaneous fibrosis |
| 0 | 3 (9%) | 1 (3%) | 1 |
| 1 | 29 (91%) | 29 (97%) | 1 |
| Dysphagia |
| 1 | 17 (53%) | 9 (30%) | 0.3 |
| 2 | 14 (43%) | 18 (60%) | 0.59 |
| 3 | 1 (3%) | 3 (10%) | 0.62 |
| Xerostomia |
| 0 | 8 (25%) | 9 (30%) | 0.6 |
| 1 | 24 (75%) | 21 (70%) | 0.76 |
Discussion
Head and neck squamous cell carcinomas are notorious for accelerated repopulation during the course of RT. This phenomenon usually sets in after four weeks of radiation therapy and to counter act this, 0.6 Gy of extra dose per day is needed [11]. To increase local control and survival, in the past decade, altered fractionation regimens have been assessed for the treatment of head and neck squamous cell carcinomas. The most commonly used altered fractionation schedules for the RT of advanced head and neck cancers are:
Hyperfractionated RT to exploit the differences in radiosensitivity of cancer and normal cells in order to increase the therapeutic ratio;
Accelerated RT to overcome tumour repopulation;
Accelerated-hyperfractionated RT to combine the effects of the two irradiation regimens.
Several prospective randomised studies have shown that accelerated RT improves loco-regional control in squamous cell carcinoma of head and neck. But accelerated regimens have been shown to increase treatment associated acute morbidity, which in severe cases might lead to an increase in late radiation effects. This study was conducted with the objective that, pure accelerated RT with concomitant chemotherapy would result in better treatment outcomes compared to conventional chemoradiotherapy. Another objective was to find out whether patients can tolerate the new accelerated schedule.
Regarding loco-regional response to RT in our study, we observed better local control both at primary and nodal site in accelerated RT arm as compared to conventional RT arm. On first follow-up, 96% had complete response at primary site and 100% had complete response at nodal site in accelerated arm and in conventional RT arm, 90% had complete response at primary site and 78% had complete response at the nodal site. This difference in loco-regional response rate was statistically significant and clearly shows a trend towards improved outcome in accelerated RT arm. At median follow-up of 17 months, loco-regional control rates were 86% in conventional RT arm compared to 90% in accelerated arm. In a prospective study by Gupta M et al., [12] at first follow-up, 90.9% had complete response at primary site and 89.1% had complete response at nodal site in accelerated arm and in conventional RT arm corresponding figures were 81.5% and 75.9%, respectively. At a median follow-up of 43 months CR was seen in 29 patients (52.7%) in the accelerated RT arm and 24 patients (44.4%) in the conventional RT arm. Though the difference in loco-regional control was not statistically significant but this study clearly indicates a trend towards improved outcome. In Danish Head and Neck Cancer Study Group (DAHANCA) study, [13] loco-regional tumour control improved significantly in the accelerated fractionation group compared with that in the conventional RT group (70% vs. 60% five years actuarial rate, p=0.0005). There was 10% statistically significant improvement in loco-regional disease control in accelerated arm. In International Atomic Energy Agency (IAEA)-ACC study by Overgaard J et al., [14] the five year actuarial loco-regional control was 42% in the accelerated versus 30% in the conventional group (p=0.004). In our study, the statistical significance could not be reached because of the small sample size and short follow-up duration.
Disease free survival in this study was slightly more in accelerated RT arm compared to conventional arm. This difference was not statistically significant (p=0.59).
We observed that acute complications were slightly more in the accelerated RT arm than those of conventional fractionation arm. Grade 2 and grade 3 skin reactions were higher in accelerated arm compared to conventional arm (89% vs. 61%; p=0.38). This was not statistically significant. Though not statistically significant, acute grade 2 and grade 3 mucositis was also higher in accelerated arm compared to conventional arm (99% vs. 75%, p=0.49). This mucositis resulted in more number of patients to become nasogastric tube dependent in accelerated RT arm compared to conventional RT (53% vs. 34%, p=0.44). In our study, higher acute reactions seen in the accelerated RT arm were expected due to accumulated dose per week (AD) of 12 Gy in accelerated arm as compared to accumulated dose per week (AD) of 10 Gy in conventional RT arm, as acute toxicity is directly dependent on accumulated dose per week. Though not statistically significant, these findings were in agreement with several other studies on accelerated fractionation. Regarding late toxicities in our study, we observed radiation induced late morbidity in the form of xerostomia, subcutaneous fibrosis at anterior aspect of neck and dysphagia did not differ significantly in both groups. Comparable late toxicities in two groups were expected as late morbidity depends upon dose per fraction which was not different in two treatment arms, that is, 2 Gy per fraction. In a study by Gupta M et al., [12] he observed that acute complications were considerably more severe in the accelerated RT arm than those of conventional fractionation arm. Grade 3 mucositis were significantly higher in the accelerated arm as compared to conventional one (63.7% vs. 19.8%; p=0.001). Moreover, the mucositis persisted longer in the accelerated fractionation arm, but all healed three months within the start of treatment. Similarly, Grade 3 and 4 skin toxicities were seen in significantly higher number of patients in the accelerated RT arm (72.7%) as compared to conventional arm (36.7%). Acute radiation morbidities were significantly higher with accelerated treatment in the 50-65 years age group because they formed the major bulk of our patients which was reflected in this study.
Most of the patients older than 65 years in accelerated fractionation suffered from Grade 3 acute radiation toxicities but it could not reach statistical significance because of small numbers. Late radiation induced morbidity was not significant in both the arms. In Danish Head and Neck cancer trial by Overgaard J et al., rate of acute radiation-related morbidity was significantly higher in the accelerated fractionation group with 53% frequency of confluent mucositis compared with 33% in the conventional treatment group (p<0.0001). Moreover, the mucositis persisted longer in the accelerated fractionation patients, but all healed within three months of the start of treatment. After five years of observation, the probability of developing severe late reaction was less than 20%. Furthermore, the probability of developing any severe late radiation-related complication, mainly in the form of late cutaneous fibrosis, mucosal atrophy, or necrosis, did not differ significantly between the fractionation groups. In another study by Overgaard J [14] (IAEA-ACC study), he observed that acute morbidity in the form of confluent mucositis was noted in 45 patients in the accelerated group and 22 patients in the conventional group (2·15, 1·27-3·35); severe skin reactions were noted in 87 patients in the accelerated group and 50 patients in the conventional group (1·91, 1·31-2·79). There were no significant differences in late radiation side-effects. [Table/Fig-8] Shows the comparison of present study with other studies.
Showing comparison with other studies [12-14].
| Criterion | Dahanca 6, 7 trials | IAEA-ACC study | Manoj Gupta et al., | Present study |
|---|
| Sample size | 726 vs. 750 | 450 vs. 458 | 54 vs. 55 | 32 vs. 30 |
| Complete response at first follow-up | | | 90% vs. 81% (NS) | 68% vs. 96% (0.03) |
| Loco-regional control | 60% vs. 70% (0.0005) after 5 yrs | 30% vs. 42% (0.004) after 5 yrs | 44.4% vs. 52.7% (0.8) after 43 months | 86% vs. 90% (NS) after 17 months |
| Acute mucositis | 33% vs.53% (0.001) | 5% vs. 10% | 16.6% vs. 32.7% (0.04) | 75% vs. 99% (NS) |
| Acute skin reactions | | 11% vs. 20% | 35% vs. 71% (0.04) | 61% vs. 59% (NS) |
| Acute dysphagia (Ryles tube dependent) | | 45% vs. 52% | 11% vs. 20.4% | 34% vs. 53% (NS) |
Limitation
The major limitations of the study were small sample size and short follow-up duration. A study with larger sample size should be conducted.
Future Recommendations
This study did not show significant difference in outcomes with accelerated fractionation regimen with concurrent chemotherapy compared to conventional concurrent chemoradiation. Though not significant, acute reactions are more in accelerated fractionation compared to conventional fractionation. This might be due to small sample size. So further studies needed to show the benefit of chemotherapy given with altered fractionation compared to conventional concurrent chemoradiation with large sample size and long duration of follow-up.
Conclusion
In view of above findings we can conclude that accelerated fractionation RT with concurrent chemotherapy has resulted in comparable loco-regional tumour control and disease free survival in patients with HNSCC with conventional fractionation at the cost of increased but tolerable acute toxicities with no evidence of increased late toxicities. Hence accelerated fractionation with concurrent chemotherapy can be considered as an alternative treatment strategy to conventional chemoradiation especially in most of Indian centres where the patient load is much higher than the facility available.