Vitamin D deficiency and insufficiency appears to be an important public health concern throughout the world [1]. Vitamin D deficiency is a global pandemic with over one billion people (25-50% of the human population) affected in all age groups and both genders [2,3]. Furthermore, vitamin D deficiency has been found to be a potential contributing cause for non-musculoskeletal chronic diseases such as cardiovascular diseases, cancer and diabetes mellitus [4,5]. Sufficient levels of the main circulating form of vitamin D, 25(OH)D is associated with substantially lower incidence rates of various type of cancers [6].
An adequate level of vitamin D is also beneficial in maintaining DNA integrity, whereas vitamin D deficiency may increase DNA damage [7]. Given this existing data, it is reasonable to hypothesise that low vitamin D status in healthy population may predispose them to diabetes and may increase DNA damage. Reports on this issue are limited and the association with DNA damage has not established in both healthy population and patients with diabetes mellitus. Thus, the main objectives of this study was to investigate the association between vitamin D and DNA damage in health and disease state (i.e., Diabetes mellitus), among subjects from Kurdistan Region.
Materials and Methods
The study was conducted in Duhok Diabetes Center, Duhok, Kurdistan Region, Iraqbetween September 2016 and March 2018. The study protocol was approved by scientific and medical ethics committee of the Duhok Collage of Medicine and Duhok Directory General of Health (References number: 07062016-4). Informed consent was obtained from all participants before commencement of study.
Study Population
The first part was a cross-sectional study carried out on 358 individuals aged 24-75 years living in Duhok Governorate, Kurdistan Region (Iraq). DNA damage of these participants was measured. Among these, 204 were type 2 diabetes patients, who consulted the diabetes center in Duhok for diabetes management, and 154 apparently healthy individuals were selected from the friends, relatives and workers of Azadi General Teaching Hospital in Duhok city. Selection of cases and healthy controls was carried out by using a systematic random sampling technique. The sample size for this study was calculated with 90% power, at a 5% level of statistical significance.
The second part was a quasi-interventional study;consisted of 65 (aged 28-65 years) subjects previously diagnosed to have low vitamin D status. Of these 44 completed the study till the last visit (25 patients with type 2 diabetes and 19 healthy individuals). Baseline and 3 months of vitamin D supplementation data were obtained.
Data Collection
A pre-tested questionnaire was designed to obtain information on gender, age (recorded to the nearest year), and weight (recorded to the nearest kilogram using a usual non-electronic scale (detectoscale), height (recorded to the nearest centimeter using the same scale), BMI was calculated for each subject. Exclusion criteria were cardiovascular, rheumatoid, renal and hepatic diseases, history of malignancy, recent infections, smoking, alcoholic and pregnancy.
Collection of Blood Samples
Participants were instructed to visit the laboratory at Department of Clinical Biochemistry, after overnight fasting for 12-14 hours and avoiding heavy physical activity for more than 2 hours before the examinations. Blood samples were collected between 9:00-11:30 am. About 10 mL of blood were withdrawn by venipuncture, using vacutainer from the antecubital vein and collected in BD Vacutainer System CAT- plain tubes The sera were separated by centrifugation using a HITACHI centrifuge (model O5P-21) at 5000 rpm for 10 minutes at room temperature and collected into two tubes, one processed immediately for measuring serum 25(OH)D by chemiluminescent emission using clinical chemistry analyserCobas 6000 Roche (open, automated, discrete and random access) which employs Vitamin D Binding Protein (VDBP) to capture both 25-hydroxyvitamin D3 and D2. This assay was intended for the quantitative determination of total vitamin D (25-OH) in human serum and plasma, and aided in the assessment of vitamin D sufficiency. The portion of liquid sera were stored at -80°C for further analysis of 8-OHdG. Measurement of 8-OHdG was done using ELISA technique (Elabsciencecata/log number E-EL-0028, USA).
Intervention Study (Vitamin D supplementation) Protocol
The effect of vitamin D supplementation on DNA damage was examined in patients with type 2 diabetes and healthy individuals with low vitamin D status 25(OH)D <20 ng/mL and high DNA damage (8-OHdG ≥4.0 ng/mL). The relative response of serum 25(OH)D was examined in 44 participants (25 patients with type 2 diabetes and 19 healthy individuals) who completed 90-day of vitamin D supplementation. All participants were instructed to takeone tablet a day of 5000 IU of Vitamin D3 [8,9]. The formula of vitamin D used was cholecalciferol from Green Field Nutrition Company (USA). About 5 mL blood samples were collected on their first visit and after 5-days post 3-months supplementation for determination of serum 8-OHdG and 25(OH)D levels.
Assessments of Studied Parameters
For proper interpretation of the results, assessment of DNA damage (8-OHdG) levels was based on a cutoff point of 4.0 ng/mL. Less than 4.0 was considered mild to moderate DNA damage, and ≥4.0 ng/mL was severe DNA damage. Assessment of Vitamin D status was done according to the pre-documented criteria, where severe deficiency was defined as <10 ng/mL, insufficiency 10-29.9 ng, sufficiency 30-150 ng/mL, and toxic ≥150 ng/mL [10]. The height and weight were recorded for each subject and the BMI was calculated by dividing body weight in kilograms by the square of height in meters. Body Mass Index (BMI) classification was done according to previously reported by Deore DN et al., [11].
Statistical Analysis
All data were analysed using the Statistical Package for Social Science SPSS version 22.0 computer software. Descriptive statistics were adapted to present data in means±SD. Independent t-test and un-paired student t-test were used to assess differences in serum analyte among groups for continues data.
Significance of association between various risk factors for categorical data was assessed by using Chi-square test for association between two groups and one-way ANOVA test for association among more than two groups. The statistical significance, direction and strength of linear correlation between 2 quantitative variables were measured by using Pearson’s correlation coefficient test. Level of statistical significance (p-value) was set at <0.05.
Results
The demographic and laboratory characteristics of the studied subjects are summarised in [Table/Fig-1]. The age, sex distribution and body mass index for both the patients and healthy individuals were nearly similar. The mean±SD of serum 8-OHdG of patients and healthy individuals was 6.04±2.73 and 3.59±2.96 ng/mL, (p<0.01). The mean±SD of serum 25(OH)D in patients was 19.97±15.10 and in healthy individuals was 23.20±13.10 (p=0.04). The mean±SD of serum 8-OHdG levels was higher among vitamin D deficiency and insufficiency patient groups compared to vitamin D sufficiency group (6.24±3.0, 6.20±2.76 and 5.10±2.1, p=0.06 respectively). A statistically significant difference was found among healthy individuals sub-groups (5.5±3.7, 3.6±3.1 and 2.8±1.5, p=0.02, respectively) [Table/Fig-2].
Demographic and laboratory characteristics of participants under study.
| Characteristics | T2DM Patients (n=204) Mean±SD | Healthy individuals (n=154) Mean±SD | p-value |
|---|
| Age (years) | 52.9±8.5 | 48.0±8.5 | 0.07 |
| Male sex {n (%)} | 73 (35.8) | 59 (38.3) | 0.55 |
| BMI (Kg/m2) | 31.36±4.5 | 29.4±6.4 | 0.05 |
| 8-OHdG (ng/mL) | 6.04±2.7 | 3.59±2.9 | <0.01* |
| 25(OH)D (ng/mL) | 19.97±15.1 | 23.2±13.1 | 0.04* |
8-OHdG:8-hydroxy-2-deoxy guanosine; 25 hydroxyvitamin D; BMI: Body mass index; Results are mean+SD; Independent t-test; *Level of significance <0.05
Serum 8-OHdG levels stratified by 25(OH)D levels.
| Vitamin D status 25(OH)D (ng/mL) | n | Mean±SD | Low-level | High-level | p-value |
|---|
| Patients |
| Deficiency (<10) | | 526.24±3.0 | 0.1 | 14.9 | |
| Insufficiency (≥10-<30) | 112 | 6.20±2.7 | 0.1 | 18.0 | 0.06 |
| Sufficiencient (≥30) | | 405.10±2.1 | 0.1 | 11.0 | |
| Healthy individuals |
| Deficiency (<10) | 12 | 5.50±3.7 | 2.0 | 11.0 | 0.02* |
| Insufficiency (≥10-<30) | 106 | 3.60±3.1 | 0.1 | 13.9 | |
| Sufficiencient(≥30) | | 362.80±1.5 | 1.0 | 6.8 | |
Results are mean+SD; one-way ANOVA test; *Level of significance <0.05
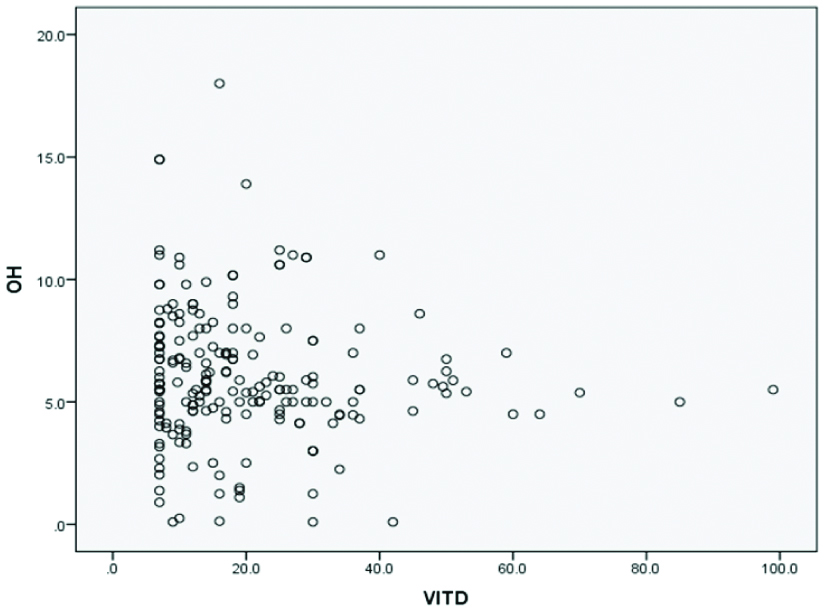
The prevalence of DNA damage according to the cutoff points is shown in [Table/Fig-3]. Of the 3 58 subjects, 217 (60.6%) were in the severe DNA damage group (8-OHdG ≥4.0 ng/mL), among them 173 (84.8%) were T2DM patients and 44 (28.6%) healthy individuals (p<0.01). Twenty-five percent of patients were in the vitamin D deficiency group, 55.0% were in the insufficiency group, and 20% had sufficient vitamin D status. The prevalence of vitamin D deficiency in healthy individuals was 7.8%, insufficiency 68.8% and sufficient 23.4%. A negative co-relation was found between 8-OHdG and 25(OH)D in the patient group (r=-0.22, p=0.01). No significant correlation was observed between 8-OHdD and 25(OH)D with respect to age and gender (r=0.04, p=0.54; r=-0.06, p=0.40, respectively) [Table/Fig-4].
Categorization of participants in 25(OH)D sub-groups.
| Parameter | Patients (n=204) n (%) | Healthy individuals (n=154) n (%) | p-value |
|---|
| 25(OH)D (ng/mL) |
| Deficiency (<10) | 52 (25.0) | 12 (7.8) | |
| Insufficiency (≥10-<30) | 112 (55.0) | 106 (68.8) | 0.01* |
| Sufficiency (≥30) | 40 (20.0) | 36 (23.4) | |
| 8-OHdG (ng/mL) |
| <4.0 | 31 (15.2) | 110 (71.4) | <0.01* |
| ≥4.0 | 173 (84.8) | 44 (28.6) | <0.01* |
Chi-square test; *Level of significance <0.05
Correlation between 25(OH)D with 8-OHdG (r=-0.22, p=0.01).
The percent changes and the significant of 8-OHdG and 25(OH)D levels at baseline and after 3 months of vitamin D supplementation are described in [Table/Fig-5]. The mean values for 8-OHdG level decreased by 13.7% (p=0.13) among patient group, associated with increased 25(OH)D level by 47.7% (p=<0.01). Regarding the healthy individuals, no such change was found in the mean level of 8-OHdG and the mean values decreased by 2.6% (p=0.36) associated with increased 25(OH)D level by 57.3% (p=<0.01).
Effects of vitamin D supplementation on 8-OHdG and 25(OH)D levels.
| Patient (n=25) | Healthy individuals (n=19) |
|---|
| Variable (ng/mL) | Pre-Supplement Mean±SD | After-supplement Mean±SD | Percent changes | p-value | Pre-supplement Mean±SD | After-supplement Mean±SD | Percent changes | p-value |
|---|
| 8-OHdG | 8.04±3.2 | 6.9±1.7 | 13.7 | 0.13 | 7.6±2.9 | 7.4±1.6 | 2.6 | 0.36 |
| 25(OH)D | 13.9±2.4 | 26.6±6.8 | 47.7 | <0.01 | 10.8±2.3 | 25.3±6.7 | 57.3 | <0.01 |
Independent t-test; *Level of significance <0.05
Discussion
The present study provides definitive evidence that high level of DNA damage in type 2 diabetes patients associated with low vitamin D levels as compared to data for healthy individuals. The results confirm a correlation between vitamin D and DNA damage, as the mean 8-OHdG levels observed in type 2 diabetes was about 2 times higher than observed in healthy individuals, and 25% of patients were in the severe vitamin D deficiency compared to 7.8% among healthy group. A negative correlation (r=-0.22, p=0.01) was observed between 8-OHdG and 25(OH)D. In the intervention study, restoration of sufficient vitamin D status after supplementation reduced DNA damage by 13.7% among patient group, this association was more evident in T2DM patient group than in healthy individuals. These results suggest that the vitamin D3 has protection effects against DNA damage, as the percentage of 8-OHdG level decreased with increasing 25(OH)D. These findings were in agreement with a similar study which suggests that vitamin D prevents DNA damage, while another study showed that increased serum 25(OH)D by the exposure to sunlight is associated with DNA damage as exposure to sun light will increase DNA damage with increasing vitamin D formation [12,13].
However, the present study is the first examinationof individual and collective effects of vitamin D on the level of DNA damage in diabetic patients and healthy individuals in Duhok, Kurdistan region (Iraq). The link between vitamin D deficiency and oxidation-induced DNA damage was studied due to high prevalence of vitamin D deficiency in our population [14]. After data analysis, we found a high prevalence (80%) of sub-optimal level of 25(OH)D (<30 ng/mL) in diabetic patients and 76.6% in healthy group. While the prevalence of DNA damage according to the suggested cut-off point (8-OHdG ≥4.0 ng/mL) in the patient group was (84.8%) and 28.6% in the healthy group. Serum 8-OHdG is known to be a sensitive marker of oxidative DNA damage and of total systemic oxidative stress in vivo [15]. Interestingly, 8-OHdG appears to play a role in tissue cell injury via the induction of apoptotic cell death [16]. It is also considered to be a measurable risk factor for co-morbid illnesses like cancer, atherosclerosis, and diabetes [17]. This may be explained by the high level of H2O2 generated from free radicals emerged from oxidative stress conditions [18]. Based on previous literature, a cut-off value of 4.0 ng/ml was used to classify the participants under study into severe DNA damage category, although some studies used different criteria [19-21]. The high level of 8-OHdG observed, suggested high rate of DNA damage which is associated with low vitamin D status [3].
Apart from vitamin D status, the DNA damage observed in T2DM patients and healthy individuals can be attributed to various confounding environment or genetic factors among our population. Further the differences in observed in results can be a scribed to different factors such as variability in sample size, differences in age range groups, environmental factors, smoking, ethnicity, and socioeconomic status including diet and other lifestyle factors, as well as supplementation period [7,22-24]. Evidence to date indicates that supplementation of population with vitamin D, or with vitamin D is in conjunction with other micronutrients, may be beneficial in some cases. The present data is in accordance with previous study by Płudowski P et al., andanimprovement in 25(OH)D levels during vitamin D supplementation at physiological doses support the existence of vitamin D deficiencies in our population [25].
Limitation
A longitudinal study may be essential to clarify the relationship between vitamin D and DNA damage among general population.
Conclusion
The present study suggests that DNA damage is prevalent in diabetic patients especially in individuals with low vitamin D status. Restoration of sufficient vitamin D status after supplementation resulted in decreased level of DNA damage by 13.7%. Preventive intervention in order to reduce the tendency of DNA damage among diabetic patients should focus on the awareness of the essentiality of vitamin D promotion of direct exposure to sunlight (vitamin D3) and consumption of vitamin D fortified food as a part of diabetic patient diet.
8-OHdG:8-hydroxy-2-deoxy guanosine; 25 hydroxyvitamin D; BMI: Body mass index; Results are mean+SD; Independent t-test; *Level of significance <0.05
Results are mean+SD; one-way ANOVA test; *Level of significance <0.05
Chi-square test; *Level of significance <0.05
Independent t-test; *Level of significance <0.05