Mucinous Adenocarcinoma of Gall Bladder- A Rare Histopathological Variant Presented as Perforation
Sunil Vitthalrao Jagtap1, Jyoti S Tele2, Snehal Rameshsing Rajput3, Swati S Jagtap4
1 Professor, Department of Pathology, Krishna Institute of Medical Sciences, Karad, Maharashtra, India.
2 Assistant Professor, Department of Pathology, Krishna Institute of Medical Sciences, Karad, Maharashtra, India.
3 Assistant Lecturer, Department of Pathology, Krishna Institute of Medical Sciences, Karad, Maharashtra, India.
4 Associate Professor, Department of Pathology, Krishna Institute of Medical Sciences, Karad, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sunil Vitthalrao Jagtap, Professor, Department of Pathology, Krishna Institute of Medical Sciences, Karad-415110, Maharashtra, India.
E-mail: drsvjagtap@gmail.com
Mucinous adenocarcinoma of gall bladder is a malignant tumour which is a very rare histopathological variant. We report a case of an 80-year-old female presented with pain in abdomen since 3 days with increasing severity and nausea since 1 day. On ultrasonography, area of altered echotexture in caudate lobe of liver was noted. Acute calculus perforated cholecystitis type II with associated peri-gall bladder collection; abscess and peri-gall bladder inflammatory changes were noted. Sub-total cholecystectomy was performed which showed perforated gall bladder with mass lesion and on histopathology, reported as mucinous adenocarcinoma of gall bladder. We are presenting this case for its rarity and clinical, radiological and histopathological findings.
Gall bladder neoplasm, Histopathology, Mucinous carcinoma
Case Report
An 80-year-old female presented with pain in abdomen since 3 days with increasing severity and nausea since 1 day. The pain was present all over abdomen. No diurnal variations noted and did not get relieved by medication. On per abdominal examination, abdomen was soft and tender. Generalised guarding was present all over the abdomen. On percussion, resonant node was felt. On auscultation, bowel sounds were normal. No organomegaly was detected. Complete blood count showed: Hb-11.1 gm%, TLC-11300/cumm. Biochemical tests showed serum total bilirubin-0.5 mg/dL, serum direct bilirubin-0.2 mg/dL, serum indirect bilirubin-0.3 mg/dL, serum alkaline phosphatase-102 IU/L, Serum Aspartate aminotransferase-13 IU/L, Alanine aminotransferase-23 IU/L. HbSAg and HCV were non-reactive. Renal functional test was normal. Clinically, provisional diagnosis was acute cholecystitis. The differential diagnosis were acute pancreatitis and acute intestinal obstruction.
On ultrasonography, pelvis-abdomen revealed area of altered echotexture in caudate lobe of liver. Rest of the wall showed diffuse thickening. Lumen showed echogenous, intraluminal abscess and debris. Break in the wall with heterogenous collection in continuity with fundus of gall bladder was noted. The hole sign was present. Impacted calculus measuring 10.6 mm at the neck of gall bladder was noted. Pancreas, spleen, kidney, urinary bladder, uterus, cervix, intestine were unremarkable. No significant abdominal lymphadenopathy was noted. On basis of clinical as well as radiological examination, diagnosis was given as acute calculus perforated cholecystitis with peri-gall bladder abscess and inflammatory changes- type II: Pericholecystic abscess and localised peritonitis. She underwent subtotal cholecystectomy. The patient was on antibiotics ceftriazone iv 1 gm bid and iv metrogyl 200 mg tid for seven days. Post-operative period was uneventful and advised follow-up.
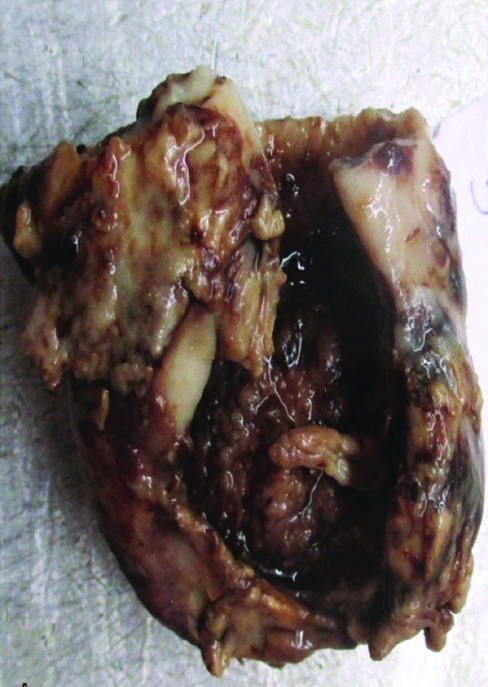
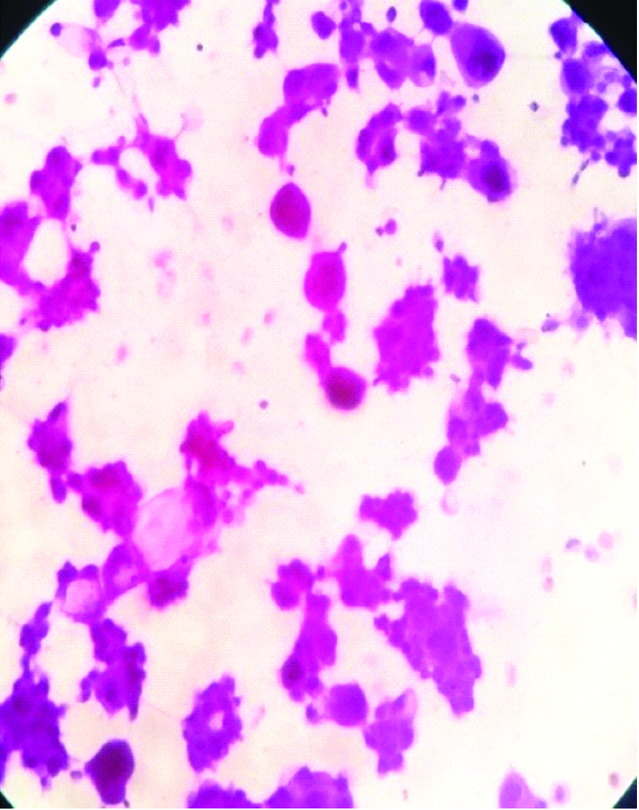
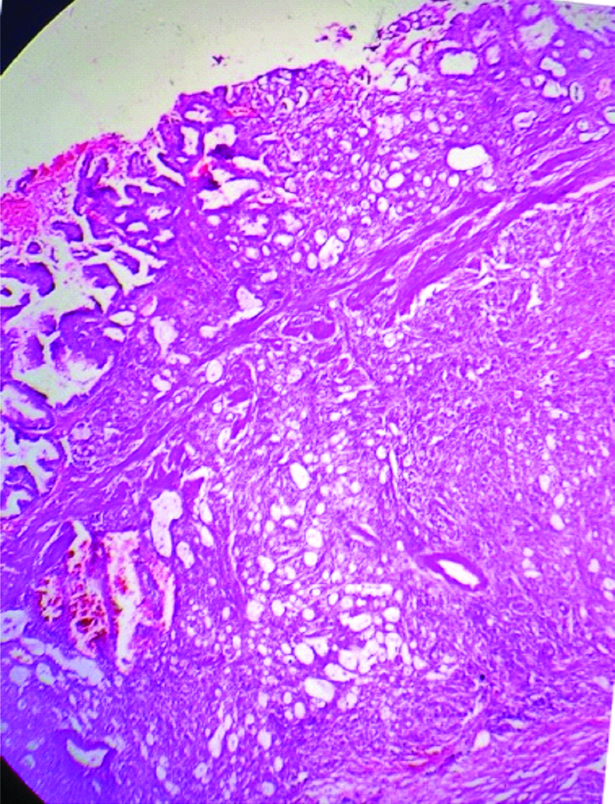
We received resected specimen of gall bladder measuring 4.5×4.2×2 cm [Table/Fig-1]. An area of perforation near fundus measuring 1.1×1.0×0.6 cm was noted. Serosal surface was rough, irregular, congested and covered with exudates. Cut open specimen revealed a large ulcero proliferative and infiltrative tumour measuring 4×3.1×1.2 cm. Cut section of tumour was shiny, soft to firm, grey white to yellow with areas of mucin. Touch imprint and mucicarmine stain was done [Table/Fig-2]. The areas of haemorrhage, necrosis were noted. An impacted calculus measuring 10.6 mm at the neck of gall bladder was noted. Tumour was seen extending upto serosa. Multiple sections from gall bladder wall showed a moderately differentiated adenocarcinoma with >60% area showing large pools of extracellular mucin with clusters of cells floating in it [Table/Fig-3,4]. The tumour cells were seen as small clusters floating in Mucin Lake, in small glandular pattern.
Gross specimen of resected gall bladder showing 4×3×1.2 cm soft, gray white tumour.
Photomicrograph of imprint, showing tumour with intra and extra cellular mucin (mucicarmine stain, x 100).
Photomicrograph of gall bladder wall showing tumour arising from mucosa and deeper invasion. (H&E stain×40).
Photomicrograph of the tumour composed of pools of extracellular mucin (small arrow) with clusters of tumour cells floating in them with few signet cells (thick arrow). (H&E stain×100).
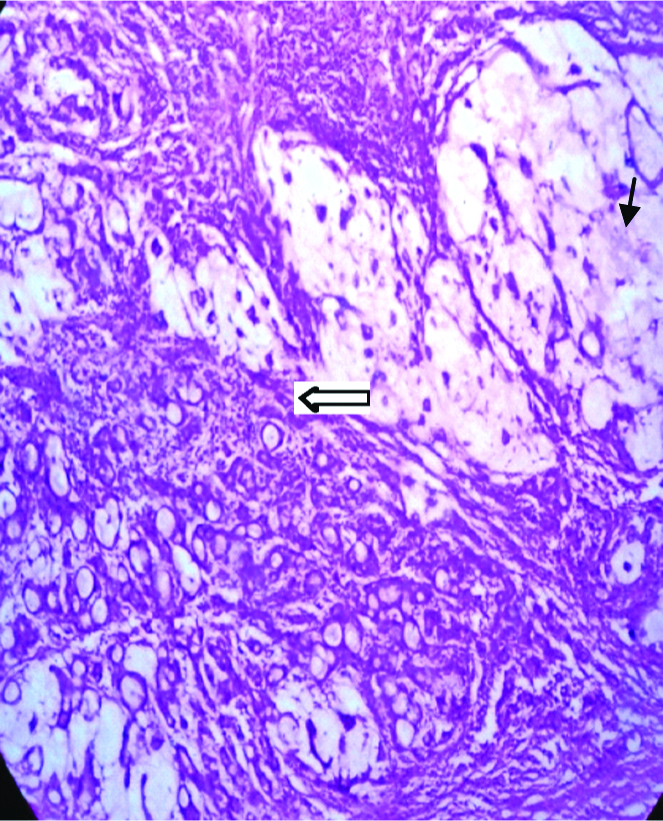
On cytomorphology neoplastic cells were having moderately pleomorphic hyperchromatic or vesicular nuclei and moderate to ample amount of cytoplasm. Stroma was loose and odematous. Few cystically dilated glands were noted. Areas of intracellular mucin, focal signet cells are noted [Table/Fig-4]. Tumour was infiltrating full thickness of gall bladder wall and extending upto serosal surface. Mucicarmine stain was positive and showed epithelial mucin in deep rose colour and nuclei black, other areas pale yellow. Areas of inflammation, congestion, necrosis and haemorrhage were noted. Rest of gallbladder showed features of acute on chronic cholecystitis with xanthogranulomas. Histopathological examination revealed gall bladder mucosa showing tumour composed of glands which are tubular complex or slightly irregular, crowded, back to back arrangement, lined by neoplastic cells having moderately pleomorphic hyperchromatic or vesicular nuclei and moderate to ample amount of cytoplasm, nuclear polarity lost. These moderately differentiated glands form carcinoma with marked desmoplasia. Extracellular mucin was abundant. The diagnosis of moderately differentiated mucinous adenocarcinoma of gall bladder was given [Table/Fig-4]. Patient was kept on regular follow-up and responded well to treatment without any complication.
Discussion
The gall bladder carcinoma is the fifth most common gastrointestinal malignancy. It is observed as a disease of elderly. It is more common in females than males. Mucinous carcinoma is very rare tumour in biliary tract and in gall bladder it constitutes about 2.5% [1]. Mucinous carcinomas are tumour diagnosed on histopathological finding in which extracellular mucin is >50% of the tumour volume [2]. The tumour cells are seen as small clusters floating in mucin lake or signet ring cells. Most of the cases are presented as acute cholecystitis. Mucinous adenocarcinoma is extremely uncommon in the gall bladder, with around 20 cases reported in the literature till date [3-5]. In 2009, WHO classify these tumours as mixed mucinous and pure (colloid) type. The Mucinous adenocarcinomas are termed when extracellular mucin constitute >50% of tumour volume and seen as mucin lakes in which neoplastic cells float as small clusters or signet cells [4]. The tumour is admixed with conventional adenocarcinoma. The pure (colloid) mucinous carcinomas are tumours in which >90% mucinous pattern is observed. Various risk factors described are gall stones, porcelain gall bladder, choledochal cyst, polypoid lesion of the gall bladder, carcinogens, sclerosing cholangitis etc., for gall bladder carcinoma. Clinically mucinous adenocarcinoma presents as abdominal pain, jaundice associated with anorexia and weight loss. The clinical presentation as gall bladder perforation is extremely rare as noted in our case.
In our case, it was type II perforation with pericholecystic abscess and localised peritonitis. The mean age group affected is 65 years with female preponderance (M:F=1:1.2) as observed by Dursan N et al., [4]. They are typically larger than adenocarcinoma with mean size around 4.8 cm. Dursan N et al., reported 15 cases of primary invasive mucinous adenocarcinoma of gall bladder in 606 cases of gall bladder carcinomas. In other organ the mucinous carcinoma are commonly noted in the intestine, breast, ovary etc., [6]. Various types of gall bladder tumours are described in which conventional adenocarcinoma constitutes >90% of cases. Mucinous adenocarcinoma constitutes about 2.5% of all gall bladder tumours. Other variants like papillary adenocarcinoma, signet ring cell carcinoma, squamous/adenosquamous cell carcinoma cribriform carcinoma, hepatoid adenocarcinoma, undifferentiated carcinoma etc., were described. In our case, it was reported as mucinous adenocarcinoma.
As per WHO criteria for mucinous adenocarcinoma >50% of extracellular mucins required for diagnosing the case [7]. The differential diagnosis are conventional adenocarcinoma with focal mucinous differentiation, metastatic intestinal carcinomas, pancreatic mucinous carcinoma. In such cases immunohistochemistry study for MVC2, MVC6, CK7/20, CDX2 tumour marker is helpful [8]. Differentiation between mucinous carcinoma of the gallbladder and pseudomyxoma peritonei is challenging. The treatment of gall bladder carcinoma is primarily surgical resection. The extension of resection depends on stage of disease. The stage beyond II, positive surgical margin, histopathologically moderate to poor tumour grade is usually associated with poor prognosis [9]. Mucinous adenocarcinoma of gall bladder is typically presented as advanced disease stage and has a poor prognosis than that of conventional adenocarcinoma [10]. The carcinoma gallbladder presenting as perforation are extremely rare to note, two cases of which were reported by Jethwani U et al., [11]. As there is paucity of cases of this rare entity, the features like recurrence and prognosis requires more studies.
Conclusion
Mucinous adenocarcinoma of gall bladder is rare histological variant. It is extremely rare to present as perforation of gall bladder which is observed in our case. We are presenting this case for its rarity, clinical course and histopathological features.
[1]. Adsay VN, Gallbladder, Extrahepatic Biliary Tree, and Ampulla. In: MillsSE, editorSternberg’s Diagnostic Surgical Pathology 2010 5th edLippincott Williams & Wilkins:1620-24. [Google Scholar]
[2]. Albores-Saavedra J, Menck HR, Scoazec JC, Soehendra N, Wittekind C, Sriram PJ, Carcinoma of the gall bladder and extrahepatic bile ducts. In: Mamilton SR, Aaltonen LA, editorsWHO Classification of tumours. Pathology and genetics of tumours of the digestive system 2000 LyonIARC Press:203-214. [Google Scholar]
[3]. Tangade AR, Rathod SG, Joshi AR, Bindu RS, Mucinous adenocarcinoma of gall bladder: a case reportInternational Journal of Research in Medical Sciences 2017 5(11):5082-84.10.18203/2320-6012.ijrms20174977 [Google Scholar] [CrossRef]
[4]. Dursun N, Escalona OT, Roa JC, Basturk O, Bagci P, Cakir A, Mucinous carcinomas of the gallbladder: Clinicopathologic analysis of 15 cases identified in 606 carcinomasArch PatholLab Med 2012 136(11):1347-58.10.5858/arpa.2011-0447-OA23106580 [Google Scholar] [CrossRef] [PubMed]
[5]. Gupte PA, Chaturvedi R, Patil LY, Joshi AS, Pure mucinous (colloid) adenocarcinoma of the gallbladder-a rare phenotypeOncol Gastroenterol Hepatol Rep 2013 2:27-29.10.4103/2348-3113.133569 [Google Scholar] [CrossRef]
[6]. Jagtap SV, Dhawan SD, Jagtap SS, Kshirsagar NS, Mucinous cystadenocarcinoma ovary presented as giant pelvic-abdominal massInt J Med Sci Public Health 2014 3(10):1305-07.10.5455/ijmsph.2014.150820145 [Google Scholar] [CrossRef]
[7]. Adsay VN, Klimstra DS, Benign and malignant tumours of the gallbladder and extrahepatic biliary tract. In: Odze RD, Goldblum JR, editorsSurgical pathology of the GI tract, liver, biliary tract, and pancreas 2009 2nd edPhiladelphiaSaunders Elsevier:857-70.10.1016/B978-141604059-0.50036-9 [Google Scholar] [CrossRef]
[8]. Giang TH, Ngoc TT, Hassell LA, Carcinoma involving the gallbladder: a retrospective review of 23 cases-pitfalls in diagnosis of gallbladder carcinomaDiag Pathol 2012 7(10):2-8.10.1186/1746-1596-7-1022284391 [Google Scholar] [CrossRef] [PubMed]
[9]. Groot KB, Fong Y, Outcome in biliary malignancyJ Surg Oncol 2014 110:585-91.10.1002/jso.2376225250887 [Google Scholar] [CrossRef] [PubMed]
[10]. Blechacz B, Gores GJ, Tumours of the bile ducts, gallbladder and ampullaGastrointestinal and Liver Disease 2010 8:1171-83.10.1016/B978-1-4160-6189-2.00069-X [Google Scholar] [CrossRef]
[11]. Jethwani U, Singh G, Mohil RS, Saroha R, Chouhan J, Bansal N, Gall bladder perforation: report of two casesOA Case Reports 2013 2(5):5010.13172/2052-0077-2-5-637 [Google Scholar] [CrossRef]