T2DM is a metabolic disorder with predisposition to CVD. CVD risk factors are associated with plasma lipid and lipoprotein abnormalities, which include dyslipidaemia. Dyslipidaemia is defined as a hyperlipidaemia condition with a high level of LDL Cholesterol (LDL-C) and reduced level of HDL Cholesterol (HDL-C) [1]. The National Cholesterol Education Program (NCEP) Expert Panel on Detection (Adult Treatment Panel III) states that HDL-C is an independent risk factor for CVD; every 1 mg/dL increment in HDL-C levels can reduce the risk for CVD by 2-3%. HDL-C levels higher than 40 mg/dL are essential [2]. However, recent studies suggest that the high levels of HDL-Cs do not specifically reduce the CVD mortality rate. In some clinical studies, patients treated with niacin or torcetrapib, did not exhibit any reduction in CVD events, although the levels of HDL-C were increased [3,4]. Therefore, assessment of CVD risk by measurement of plasma HDL-C concentration remains debatable.
LDL is a major risk factor for CVD. LDL, when oxidised (ox-LDL) is not taken up by LDL receptors but rather by macrophages via scavenger receptors to form foam cells, which accumulates in the arterial wall [5]. LDL particles isolated from normal and diabetic patients varies in their lipid compositions. Diabetic LDL comprises more TG content and is more prone to oxidise than non-diabetic individuals as reported by Seghrouchni I et al., [6]. Thus, individuals with diabetes have characteristic atherogenic dyslipidaemia with decreased HDL-C, elevated TG and increased susceptibility to LDL oxidation [7,8].
The cardioprotective effect of HDL is reverse cholesterol transport, in which the cholesterol deposited in peripheral tissues and lipid laden macrophages (foam cells) are returned to the liver. This process provides an efficient pathway for the removal of excess cholesterol from peripheral tissue and excretion of metabolised cholesterol into the bile. As a result, decreased intracellular cholesterol reduces the risk of incidence of CVD [9]. Another important function of HDL in reducing CVD events is its antioxidant activity. An important antioxidant enzyme associated with HDL particles is PON1. PON1 is synthesised by the liver and circulates in the blood in coordination with the HDL apolipoprotein (apo) A-I [10]. PON1 hydrolyses hydrogen peroxide and lipid peroxide that are either free or present in atherosclerotic lesions or in minimally oxidised LDL [11]. HDL associated PON1 have protective action towards LDL oxidation and reduced PON-1 activity has been reported in many diseases, such as CVD, T2DM and chronic kidney failure [10]. T2DM patients are highly susceptible to CVD development due to decreased HDL-C. Kalmár T et al., reported reduced serum PON1 activity in T2DM as compared to the healthy population, while HDL antioxidant capacity was not observed [12].
Based on these findings, the present study was designed to assess the susceptibility of LDL particles to Cu2+-mediated oxidation and the antioxidant activity of HDL against LDL oxidation in-vitro in T2DM patients and non-diabetic control subjects. The study also aimed to evaluate the correlation between serum PON-1 activity and percentage inhibition of HDL against LDL-oxidation.
Materials and Methods
Participants
This cross-sectional study was conducted from November 2013 to July 2014 in the Health Sciences Service Unit, Faculty of Allied Health Sciences, Chulalongkorn University, Thailand. The sample size, 20 T2DM and 20 healthy controls, was calculated by the G*power3 program. The 80% power of detection differences comprises the means versus the alternative of equal means. The size of the variation in the means was represented by their standard deviation alpha error (0.5). This research was approved by the Research Ethics Review Committee for research Involving Human Research Participants, Health Sciences Group, Chulalongkorn University (Certificate of Approval number 061/2552).
Inclusion criteria for T2DM patients were aged between 30-60 years, fasting blood glucose ≥126 mg/dL, and HbA1c≥6.5 mg/dL [1]. Age and gender-matched, 20 apparently healthy subjects were recruited as control. The inclusion criteria for control were as follows: fasting total cholesterol <220 mg/dL; triglyceride <150 mg/dL; fasting blood glucose <110 mg/dL; and haemoglobin A1c (HbA1c) <6.0 mg/dL [13]. The exclusion criteria were as follows: no underlying diseases such as CVD, kidney disease, liver disease, cancer and/or in the process of chemotherapy; smoking or drinking alcohol regularly; taking food supplements or vitamins within 1 month before participation. Written informed consent was obtained from all participants after complete explanation of the purpose, nature and potential risks of this study.
Blood collection: Overnight fasting blood samples (10 mL) were collected in EDTA and NaF coated vacutainer. Serum and plasma were intermediately separated by low-speed centrifugation at 3000×g 4°C for 10 minutes and stored in aliquots at -80°C until further analysis. Fasting blood glucose and HbA1c were determined using an Automated analyser (Cobas e411).
Lipoprotein separation: Lipoproteins were isolated from plasma by sequential ultracentrifugation (Microultracentrifuge, himac CS 150GXL, Hitachi, Japan) [14]. Very low-density lipoprotein (VLDL), LDL and HDL were isolated in a density range of 1.006-1.019 g/mL, 1.019-1.063 g/mL and 1.063-1.21 g/mL, respectively. TC and TG concentrations in each lipoprotein fraction were measured by enzymatic colourimetric method [15,16]. Commercial reagents were purchased from Human, Germany and the absorbance was measured using an Evolution 300 Spectrophotometer (Thermo Fisher Scientific, USA).
Serum paraoxonase-1 activity: Serum PON1 activity was measured by using 5.5 mmol/L paraoxon as a substrate (Sigma-Aldrich, USA). The change in absorbance of p-nitrophenol was measured at 412 nm using a Microplate Reader Power Wave 340 (BioTek, UK). The results were expressed as units per mL of serum. A unit (U) of PON1 activity was defined as 1 nmol of p-nitrophenol formed per minute [17].
Antioxidant activity of HDL against LDL oxidation, in vitro: The inhibitory activity of HDL was determined by co-incubation of HDL isolated from participants with LDL isolated from a single healthy donor (control LDL, cLDL). The lipid peroxides generated during oxidation were measured in three different conditions: cLDL alone, HDL (isolated from T2DM or control) alone, and cLDL+HDL. c-LDL (50 μg protein/mL), HDL (100 μg protein/mL) and LDL+HDL (50+100 μg protein/mL) were oxidised in PBS at 37°C in the presence of 5 μM CuSO4 in a total volume of 0.5 mL. Lipid peroxide was measured at 0, 1, 2 and 3 hours after incubation. The reaction was terminated with 90 μM butylated hydroxytoluene (final concentration) [6].
Susceptibility of LDL to oxidation in vitro: LDL was desalted with 0.1M Phosphate Buffered Saline (PBS) (pH 7.4) by gel filtration on Sephadex G-25M PD-10 columns (Amersham, USA). The protein concentration in the purified lipoprotein fraction was measured by the Lowry method using bovine serum albumin as the standard [7]. LDL (50 μg protein/mL) isolated from T2DM and the control group was oxidised by 5 μM CuSO4 in PBS at 37°C for three hours. Aliquots were taken for lipid peroxide determination at 0, 1, 2 and 3 hours after oxidation. The reaction was terminated with 90 μM butylated hydroxytoluene (BHT) (final concentration) [18].
Lipid peroxide measurement: Lipid peroxides generated by oxidation from previous experiments were measured by Naurooz-Zadeh J et al., [8]. In brief, 100 μL of solution collected from every oxidation experiment was mixed with 900 μL of FOX-2 reagent (250 μM ammonium ferrous sulphate, 100 μM xylenol orange, 25 mM H2SO4, 4 mM butylated hydroxytoluene in 90% (v/v) methanol), for 30 minutes at room temperature. The absorbance of the samples was read at 560 nm against a H2O2 standard curve, which was linear in the range 0-20 μM.
The percent inhibition of LDL oxidation by HDL was calculated according to the following equation [18]:
Inhibition of HDL against LDL oxidation (%)=(cLDL+HDL)-LH×100 cLDL+HDL
Where; (cLDL+HDL) is the sum of the lipid peroxide generated from cLDL and HDL when incubated separately with 5 μM CuSO4 at 0, 1, 2 and 3 hours, while LH is the lipid peroxide generated during co-incubation of cLDL with HDL and 5 μM CuSO4 at 0, 1, 2 and 3 hours.
Statistical Analysis
The results are reported as the mean±SD. Data were analysed by Statistical Package for the Social Sciences (SPSS) statistical software (Version 14.0). The significant differences between the T2DM group and control group were analysed using an independent samples t-test. The correlation between percentage inhibition of HDL and serum PON1 activity was analysed by simple linear regression. The p-values <0.05 were considered statistically significant.
Results
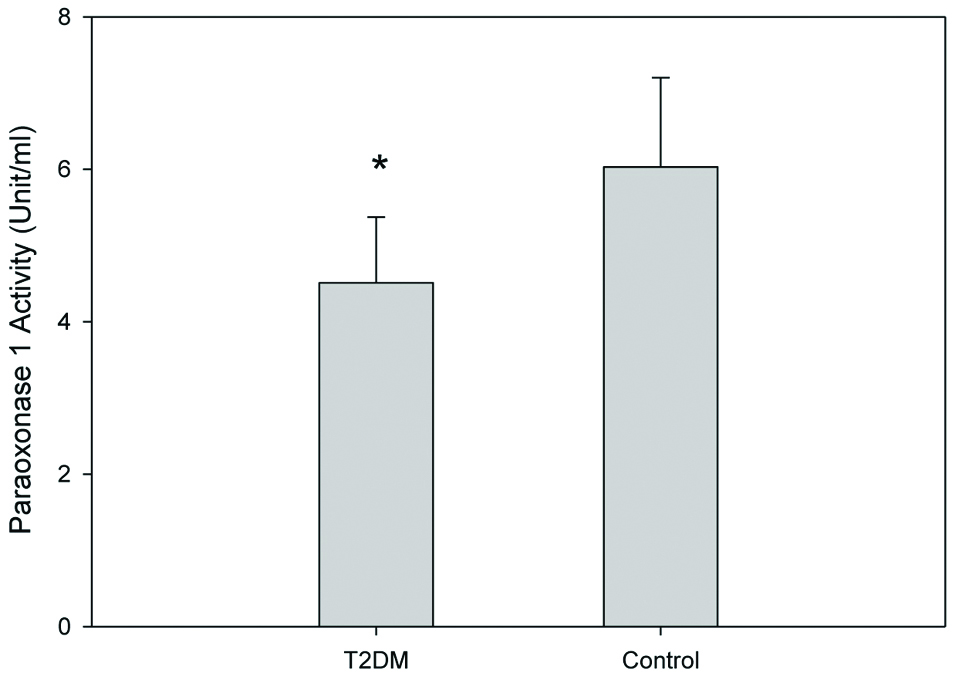
The general characteristics of all participants were reordered and are shown in [Table/Fig-1]. T2DM group showed a significant increase in fasting blood sugar and HbA1c (p-value <0.05), as compared to the control group. Lipoprotein profiles of the T2DM group and the control group was measured [Table/Fig-2]. TC, VLDL-C and LDL-C concentrations in T2DM were significantly increased, whereas HDL-C levels were marginally decreased. TG concentration in plasma, VLDL, LDL and HDL were also significantly increased in the T2DM group. Serum PON1 activity was also investigated in the T2DM and control groups. Serum PON1 activity was reduced in T2DM patients compared to the control group [Table/Fig-3].
General characteristics of T2DM and control participants under study.
| T2DM (n=20) | Control (n=20) | p-value |
|---|
| Sex (Male/female) | 12/8 | 12/8 | |
| Age (years) | 46±6 | 42±5 | 0.25 |
| Fasting blood sugar (mg/dL) | 176±52 | 89±6 | 0.01* |
| Hemoglobin A1c (HbA1c) | 8.8±1.4 | 5.3±0.3 | 0.04* |
Data are mean±SD; p-value of an independent samples t-test; *p< 0.05 is statistically significant; T2DM: Type II diabetic patients
Lipoprotein profiles of T2DM patients and control participants under study.
| Lipoprotein profiles (mg/dL) | T2DM (n=20) | Control (n=20) | p-value |
|---|
| Total Cholesterol | 212.5±10.5 | 182.4±5.4 | 0.04* |
| VLDL-C | 41.5±13.0 | 30.7±10.3 | 0.04* |
| LDL-C | 99.8±15.8 | 69.5±6.7 | 0.03* |
| HDL-C | 43.5±7.4 | 62.60±5.5 | 0.03* |
| Triglycerides (mg/dL) | 223.60±16.4 | 79.1±7.5 | 0.008* |
| VLDL-TG | 142.1±57.2 | 43.3±25.8 | 0.007* |
| LDL-TG | 50.5±2.3 | 12.5±2.2 | 0.007* |
| HDL-TG | 22.6±2.81 | 12.5±2.5 | 0.01* |
Data are mean±SD; p-value of an independent samples t-test; *p< 0.05 is statistically significant; T2DM: Type II diabetic patients; VLDL-C: Very low-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; VLDL-TG: Very low-density lipoprotein triglyceride; LDL-TG: Low-density lipoprotein triglyceride; HDL-TG: High-density lipoprotein triglyceride
Serum PON1 activities (unit/ml) of T2DM and control group. Results are expressed as units per ml of serum.
*Independent samples t-test; significant difference from control; p=0.035; T2DM: Type II diabetic patients
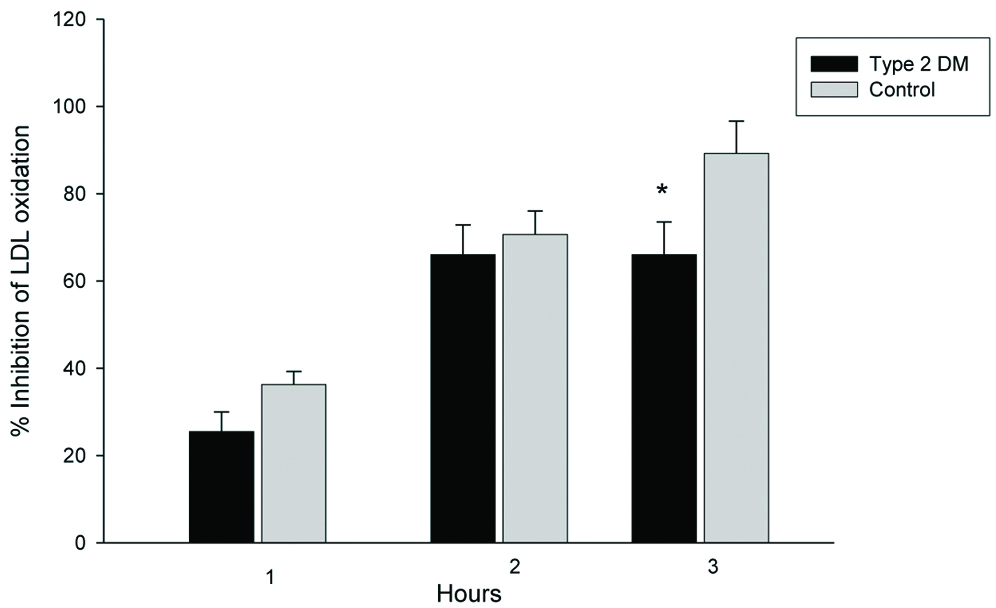
Percent inhibition of HDL against LDL oxidation was examined. Control LDL was oxidised in the presence or absence of HDL in vitro. The lipid peroxide generated from cLDL during oxidation was detected by FOX2 reagent. The ability of HDL antioxidation was expressed as percentage inhibition. The inhibitory activities of HDL isolated from T2DM patients decreased. Using lipid peroxide as a marker for oxidation, HDL inhibited cLDL oxidation by 25.4±4.5, 66.0±6.8, and 66.0±7.5% at 1, 2 and 3 hours respectively, in the T2DM group. The inhibitory activities of control HDL on LDL oxidation were 36.3±3.0, 70.6±5.4, 89.2±7.4% at 1, 2 and 3 hours respectively [Table/Fig-4]. Percent inhibition of HDL against LDL oxidation isolated from T2DM patients was marginally lower (not significant) than control HDL at 1 and 2 hours after oxidation. After 3 hours of oxidation, percentage inhibition of HDL isolated from T2DM was significantly lower than control HDL.
Inhibitory activity of HDL on LDL oxidation in vitro. T2DM (n=20) and control (n=20) HDLs (100 μg protein/mL) were co-incubated with LDL (50 μg protein/mL) isolated from a single healthy donor.
*Independent samples t-test, significant difference from control, p=0.04
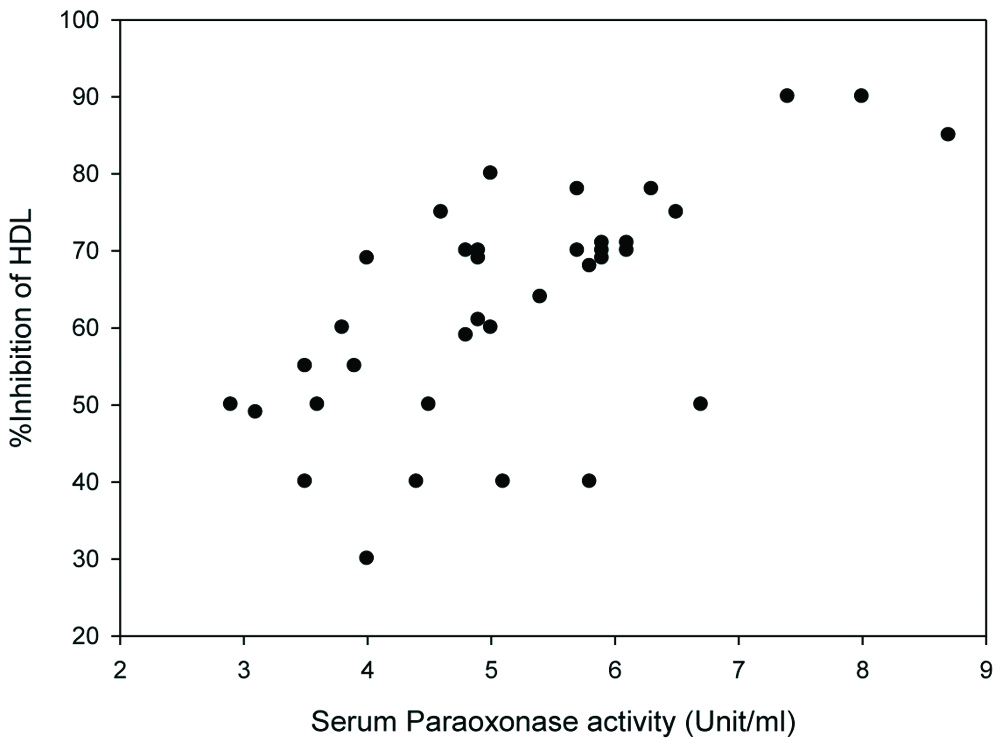
The correlation between PON1 activity and percentage inhibition of HDL against LDL oxidation was observed. Percent inhibition of HDL against LDL oxidation from T2DM patients and control subjects were plotted against serum PON1 activity. Positive correlation was observed between percentage inhibition of HDL against LDL oxidation and serum PON1 activity, with r-value=0.64 (p-value <0.01) [Table/Fig-5].
The correlation between serum PON1 activity (X-axis) and percentage inhibition of HDL against LDL oxidation (Y-axis). The correlation coefficient r-value was 0.64, p<0.01.
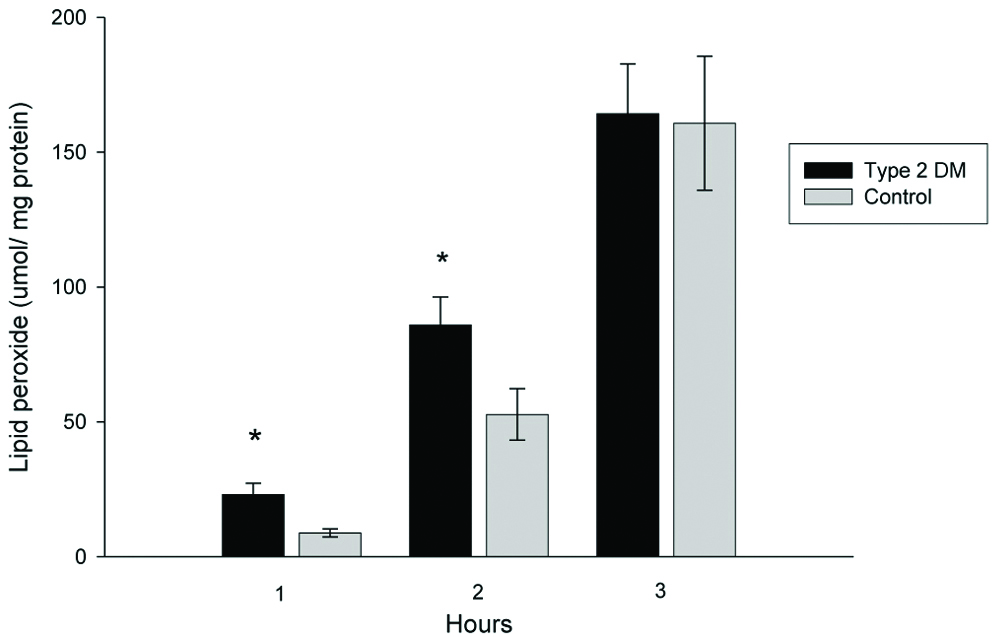
LDL susceptibility to oxidation in vitro was evaluated by measuring lipid peroxides at 1, 2 and 3 hours after oxidation [Table/Fig-6]. The amount of lipid peroxide generated from LDL during copper-induced oxidation was increased in T2DM patients. A significant difference was found at two hours after oxidation; T2DM LDL generated lipid peroxides by 85.9±10.3 μmol/LDL mg protein, whereas, normal LDL generated lipid peroxides by 52.7±9.5 μmol/LDL mg protein, p<0.05. Lipid peroxides generated from T2DM patients’ LDL oxidation showed an increasing trend (not significant) at 1 and 3 hours after oxidation compared to the control group.
Lipid peroxide generated from LDL oxidation.
*Independent samples t-test, significant difference from control, p <0.05
Discussion
The cardioprotective properties of HDL have been attributed to inverse cholesterol transport. Recently, researchers have found that HDL has the ability to directly inhibit LDL oxidation in vitro [21,22]. T2DM is commonly associated with dyslipidaemia, including hypertriglyceridemia, and the low levels of HDL-C that are risk factors for CVD [7,8]. The present study aimed to assess the HDL antioxidant activity in T2DM and non T2DM participants. The study also correlated HDL antioxidant activity and serum PON1 activity.
HDL are known to have an anti-atherogenic function. They mediate cholesterol efflux from macrophages in the vessel wall, an important role in the maintenance of net cholesterol balance in the arterial wall. Another important HDL function is anti-oxidation. HDL antioxidant activity is mediated by Apo AI coordinating enzyme, such as PON1, platelet-activating factor acetylhydrolase and glutathione peroxidase. PON1 is highly effective in preventing LDL oxidation and is synthesised in the liver, transported into the blood and circulated by association to Apo-AI in HDL particles [23,24,25]. PON1 concentration and activity are reduced in T2DM, which contributes to the development of CVD in T2DM [25,26]. Furthermore, PON1 concentration decreases as the duration of diabetic condition increases, especially in an uncontrolled diabetes and dyslipidaemia [27]. The reduction in PON1 activity and HDL-C levels increases risk of CVD development in T2DM patients. Therefore, it was proposed that the CVD risk assessment model should evaluate specific HDL functions, such as antioxidant activity, which may be a better indicator than HDL-C concentration for predicting CVD risk factors.
In the present study, the T2DM group was hyper-triglyceridemia (mean of plasma TG=223 mg/dL), whereas the control group was normo-triglyceridemia (mean of plasma TG=79 mg/dL). VLDL particles isolated from T2DM were rich in TG (VLDL-TG=142 and 43 mg/dL in T2DM and control VLDL, respectively, p-value=0.007). These findings were in accordance with the lipid profile observed by Krauss RM et al., [7]. TC in plasma and all lipoprotein fractions were significantly increased in T2DM patients, which is similar to the Gordon L et al., findings [25]. Insulin resistance found in T2DM patients stimulates fatty acid secretion into the liver and promotes VLDL synthesis. Lipoprotein lipase, an enzyme that hydrolyses TG from VLDL, is dependent on insulin action. In insulin resistance, TG hydrolysis from VLDL is affected that leads to hyper-triglyceridemia. The VLDL particles in the insulin resistance stage are TG-rich particles, and these particles are considered to be atherogenic VLDL [28]. In lipoprotein metabolism, the TG and Cholesteryl Ester (CE) in lipoprotein can be exchanged. The Cholesteryl Ester Transfer Protein (CETP) transfers CE from LDL or HDL to VLDL particles, and TG is exchanged with CE and moves to LDL and HDL particles. After lipid transfer, LDL and HDL become TG-rich LDL and TG-rich HDL and VLDL particles become CE-rich VLDL [29,30]. The present results depicts that the LDL and HDL isolated from T2DM showed significantly increased TG concentration compared with the control group. TG-rich HDL cannot circulate in the blood longer time; and are rapidly removed in the liver, resulting in the reduction of plasma HDL levels [21]. The findings of the present study confirmed the reduced HDL-C in T2DM patients, leading to increased risk of CVD.
HDL reduction is commonly found in T2DM patients, but HDL function is also an important factor for evaluating the CVD risk factor. In the present study, authors observed that HDL isolated from T2DM patients was less effective than control HDL in inhibiting LDL oxidation. The percentage inhibition of HDL isolated from T2DM patients was significantly lower than control HDL at 1, 2 hours after oxidation. This finding is consistent with the results of Morgantini C et al., study [27]. These results herein support the hypothesis that HDL isolated from T2DM patients is impaired in antioxidant activity. The antioxidant activity of HDL is mediated by PON1, an Apo-A1 co-ordinating enzyme. Serum PON1 activity is a marker for antioxidant status. In the present study, the serum PON1 activity in T2DM patients was significantly reduced compared to the control, which is comparable to results of Viktorinova A et al., and Wamique M et al., [27,30]. The correlation between serum PON1 activity and percentage inhibition was also investigated. Percent inhibition of HDL against LDL oxidation was proportional to serum PON1 activity. The combination of low HDL-C concentration and reduced PON1 activity in T2DM may stimulate a CVD event.
LDL particles are small and can penetrate into the arterial wall. LDL can be oxidised in the arterial wall, non-enzymatically by metal ions or hemin. It may also be exposed by cell-derived oxidants in subendothelial cells [31]. Lipid soluble vitamins contained in LDL particles include vitamin E, which acts as an antioxidant. In the early oxidation stage, vitamin E in LDL particles can protect the damage of lipids by oxidative stress. Continuing oxidative damage may consume the vitamin E in LDL particles until it is exhausted [32]. After this stage, lipids contained in LDL are modified and oxidised. These fully oxidised LDL enters the blood circulation and therefore can be used as a marker for CVD patients [32]. Holvoet P et al., studied ox-LDL levels in patients with acute coronary syndrome; stable angina, unstable angina and acute myocardial infarction [33]. They reported that ox-LDL concentrations were higher in patients with coronary syndrome compared to control. Furthermore, the circulating ox-LDL significantly correlated with the Global Risk Assessment Scoring (GRAS; based on age, TC, HDL-C, systolic blood pressure, diabetes mellitus, and smoking); signifying that the circulating ox-LDL may predict CVD events [34]. The increase in circulation fox-LDL in T2DM was reported by Nijajou OT et al., [35]. In the present study, LDL isolated from T2DM was more susceptible to oxidation than control LDL. The lipid peroxides generated from LDL oxidation were higher than control LDL at 1 and 2 hours after oxidation. Although the serum ox-LDL was not observed, it is implied that the serum oxidised LDL will be higher in T2DM than the control group.
The atheroprotective function of HDL includes reverse cholesterol transport, antioxidation, anti-inflammation, anti-platelet aggregation etc. Some patients develop CVD even though their HDL-C levels are in the normal range. This evidence reveals that the HDL-C measurement is not sufficient to evaluate the cardioprotective potential of HDL. Specific functions may be better indicators than HDL-C. Antioxidant activity of HDL is one of many steps protecting LDL from oxidation in early atherosclerotic process and therefore, antioxidation activity of HDL testing should be applied in the routine laboratory, especially in T2DM patients.
Limitation
Although this research evaluated the antioxidant activity, but HDL exhibits many anti-atherosclerotic activities, like reverse cholesterol transport, anti-inflammatory or anti-platelet aggregation. These should also be done.
Conclusion
HDL isolated from T2DM patients showed impaired antioxidant activity against LDL oxidation, while serum PON1 activity was diminished in T2DM. Furthermore, LDL separated from T2DM patients was found to be susceptible to in-vitro oxidation. These characteristics tend to promote CVD events in T2DM patients. The data in this study suggest that the risk assessment for CVD should add a qualitative evaluation for HDL function, such as antioxidant activity, to the conventional quantitative measurement of HDL-C.
Data are mean±SD; p-value of an independent samples t-test; *p< 0.05 is statistically significant; T2DM: Type II diabetic patients
Data are mean±SD; p-value of an independent samples t-test; *p< 0.05 is statistically significant; T2DM: Type II diabetic patients; VLDL-C: Very low-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; VLDL-TG: Very low-density lipoprotein triglyceride; LDL-TG: Low-density lipoprotein triglyceride; HDL-TG: High-density lipoprotein triglyceride