Coronary Artery Disease (CAD) is one of the top causes of mortality and morbidity worldwide. CAD is the major cause of death for person aged 35 and over [1-3]. Indonesian Ministry of Health research showed that CAD is responsible for 12.9% of the mortality [4]. In patients with CAD, EPCs proliferation and migration capability are significantly reduced as the disease progresses. Thus, further reducing EPCs capability to repair the vascular damage [5,6]. Patients with low EPCs count and impaired migration activity also have a higher incidence of cardiovascular events, mortality, and morbidity compared to patients with higher EPCs count and migration [7]. Multiple pathways is suggested to be responsible for EPCs impairment in the CAD patients. It is suggested that oxidative stress play significant roles in EPCs impairment through intracellular damage and redox balance disruption [8]. Disruption of intracellular redox environment has a critical role in controlling apoptosis, proliferation, self-renewal, senescence, and differentiation of EPCs thus predisposing to the development of vascular pathology [9].
Statins or HMG-CoA reductase inhibitors have been well established as safe and effective treatment in reducing cardiac event through decrease of serum LDL-cholesterol [10]. However, recent studies show that statins have early beneficial effect for coronary heart disease before any significant reduction in lipid profile. This suggests that statins’ benefit to reduce cardiac event may occur via alternative mechanism other than LDL-c reduction alone [11]. Improvement of EPCs proliferation and migration has been proposed as alternative mechanism of statins to reduce cardiac event. Simvastatin is able to enhance thioredoxin enzymatic activity, resulting in a significant reduction in intracellular reactive oxygen species [12]. Simvastatin and rosuvastatin have been proven to increase EPCs proliferation and migration through activation of the endothelial Nitric Oxide Synthase (eNOS) pathway, which can increase bioavailability of Nitric Oxide (NO) that can stimulate soluble kit ligand release that induce EPCs migration [13,14]. Atorvastatin also proven to increase the amount of EPCs proliferation and migration through a mechanism that was independent of plasma levels of cytokines and cholesterol [15]. However, comparison between each statin toward EPCs migration is yet to be investigated. Herein, in this study, authors evaluated and compared the effect of simvastatin, atorvastatin and rosuvastatin treatment toward migrational capabilities of EPCs from CAD patients.
Materials and Methods
The study was a cohort study conducted from June 2018 to December 2018 in Dr. Soetomo General Hospital, Surabaya, Indonesia. Blood samples were obtained from sequential sampling of eight CAD patients from Department of Cardiology. The patients were male, aged 40-59 years, diagnosed with stable angina with >50% stenosis on Left Main Coronary Artery (LMCA) or >70% on other coronary artery. Patient with history of Percutaneous Coronary Intervention (PCI), Coronary Artery Bypass Grafting (CABG), new onset acute myocardial infarct, diabetes and anaemia were excluded.
This study had received ethical clearance from Local Ethic Committee from Dr. Soetomo General Hospital (0379/KEPK/VII/2018). All patients gave written informed consent prior to peripheral blood drawing. Details which disclose patient’s identity of the donor patients were omitted.
EPCs Isolation and Culture
Blood samples were collected from ante-acubital vein using a sterile needle and syringe. To separate EPCs from Peripheral Blood Mononuclear Cells (PBMCs), authors used a standard protocol [16]. PBMCs were obtained by Ficoll Histopaque1077 (Sigma-Aldrich, USA). PBMCs at the concentration of 5×105 cells/mL, supplemented with 15% FBS, 40 ng/mL of Vascular Endothelial Growth Factor (VEGF) in basal stemline II Haematopoietic Stem Cells (HSC) expansion medium (Sigma-Aldrich, USA), were seeded onto the fibronectin-coated six-well plate. Culture was maintained at 37°C with 5% CO2 with humidified atmosphere. Two days after, non-adherent cells were discarded and replaced by fresh medium. The medium was changed twice a week to ensure optimum culture condition. EPCs were confirmed using FITC-labeled Anti-human CD34 antibody (Biolegend, USA) through immunofluorescence examination.
Endothelial Progenitor Cells (EPCs) Treatment
After 3 days, EPCs culture was divided into four group of treatment: 1) Negative control; 2) Simvastatin 0.5 μmol/L; 3) Atorvastatin 0.5 μmol/L; 4) Rosuvastatin 0.5 μmol/L. Cell culture was incubated for 48 hours and quadruplicate cell wells were used for each data point.
Migration Assay
Boyden chamber assay method was used to calculate EPCs migration [17]. Isolated EPCs were detached using 1 mmol/L EDTA in PBS, and centrifuged 400× for 10 minutes at room temperature, then, a total of 5×105 EPCs were placed in modified Boyden chamber at the upper chamber. A 2 mL of basal media was added in the lower chamber compartment followed by 24 hour incubation at 37°C. Non-migratory cells were removed manually. New basal medium was placed on the receiver plate and added 500 μL of trypsin+EDTA solution 0.5% and incubated for 10 minutes. Verification was done by using a light microscope to observe if the cell that was released >90%. The cell on the receiver plate then inserted into a 15 mL conical tube, then harvested and counted using an automated cell counter with trypan blue colouring.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics 25.0 from IBM. Data were considered significantly difference if p<0.05. Continuous variables, presented as mean±SD, were evaluated for normal distribution and compared using the ANOVA test, as appropriate. Correlation between parametric variables was obtained using pearson correlation followed by linear regression test.
Results
Demography of the CAD Patients
Blood sample was obtained from eight CAD patients with demography as presented in [Table/Fig-1].
Characteristic of the CAD patients.
| Variable | Mean±SD |
|---|
| Age (years) | 54.50±4.31 |
| Height (cm) | 168.0±1.30 |
| Weight (kg) | 70.25±6.34 |
| BMI (kg/m2) | 25.39±2.13 |
| Systolic blood pressure (mmHg) | 137.50±24.35 |
| Diastolic blood pressure (mmHg) | 80±7.56 |
| Heart rate (times/minute) | 86±8.68 |
| Total cholesterol (mg/dL) | 200.50±74.75 |
| Triglyceride (mg/dL) | 97±11.64 |
| LDL (mg/dL) | 145±61.11 |
| HDL (mg/dL) | 35±7.64 |
| Left ventricle ejection fraction (%) | 53.5±4.11 |
Statins Increases EPCs Migration in Dose-dependent Manner
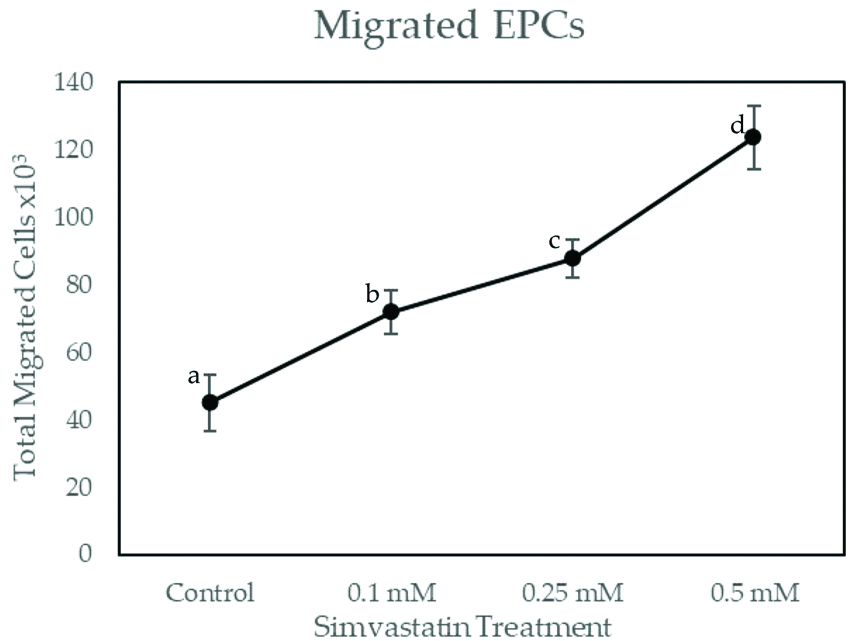
As shown in [Table/Fig-2], total migrated EPCs on simvastatin dose 0.1 mM, 0.25 mM and 0.5 mM have significant differences with the control group (p<0.001). The number of migrated cells in control group was 45,000.00±8,215.84. EPCs migrations were increased in dose-dependent manner, which were 72,000.00±6,363.96 for 0.1 mM simvastatin, 87,750.00±5,809.47 for 0.25 mM simvastatin and 123,750.00±9367.49 for 0.5 mM simvastatin. Pearson’s correlation showed significant and very strong correlation between simvastatin treatment with the number of migrated EPCs (r=0.964; p≤0.001). Linear regression test showed r-square of 0.929. This suggested that simvastain treatment was responsible for 92.9% of EPCs migration.
Total Migrated EPCs on Increasing Dose of Simvastatin Treatment.
EPCs were exposed to increasing concentrations of simvastatin for 48 hours. Migrated EPCs was determined via Boyden Chamber Assay. Total migrated cells were expressed as mean±SE (n=4). Different annotation (a,b,c,d) denounce significant difference in ANOVA test (p<0.001)
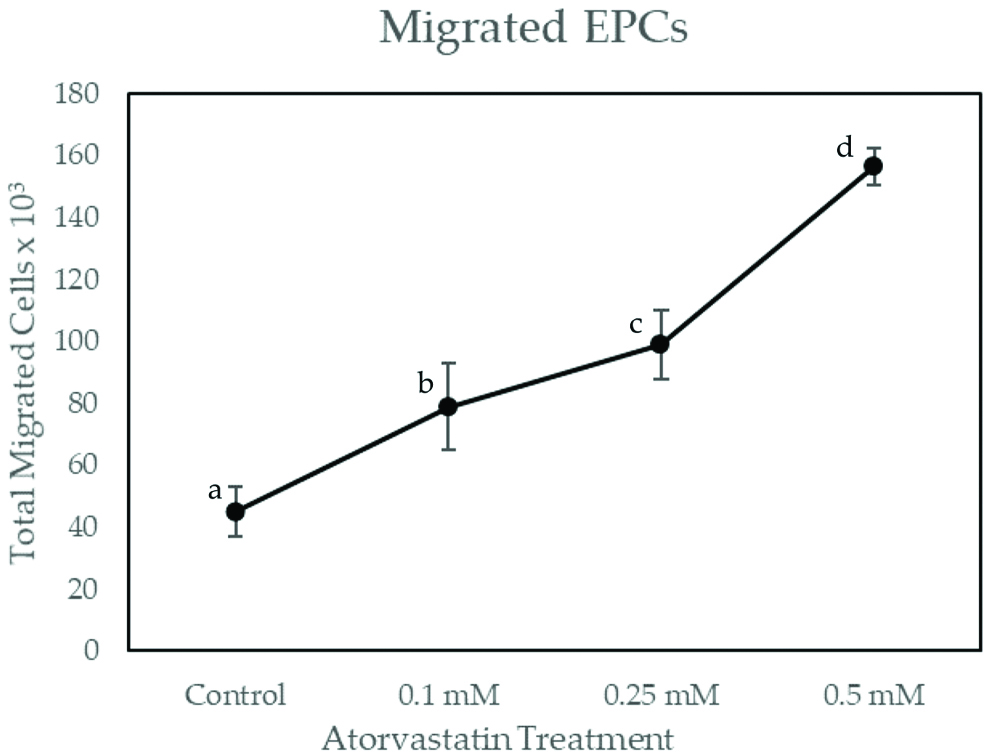
As shown in [Table/Fig-3], total migrated EPCs on atorvastatin dose 0.1 mM, 0.25 mM and 0.5 mM have significant differences with the control group (p<0.001). EPCs migrations were increased in dose-dependent manner, which were 78,750.00±7,794.22 for 0.1 mM atorvastatin, 99,000.00±8,215.83 for 0.25 mM atorvastatin and 156,375.00±12,392.03 for 0.5 mM atorvastatin. Pearson’s correlation showed significant and very strong correlation between simvastatin treatment with the number of migrated EPCs (r=0.869; p≤0.001). Linear regression test showed r square of 0.755. This suggested that atorvastatin treatment is responsible for 75.5% of EPCs migration.
Total Migrated EPCs on Increasing Dose of Atorvastatin Treatment.
EPCs were exposed to increasing concentrations of atorvastatin for 48 hours. Migrated EPCs was determined via Boyden Chamber Assay. Total migrated cells are expressed as mean±SE (n=4). Different annotation (a,b,c,d) denounce significant difference in ANOVA test (p<0.001)
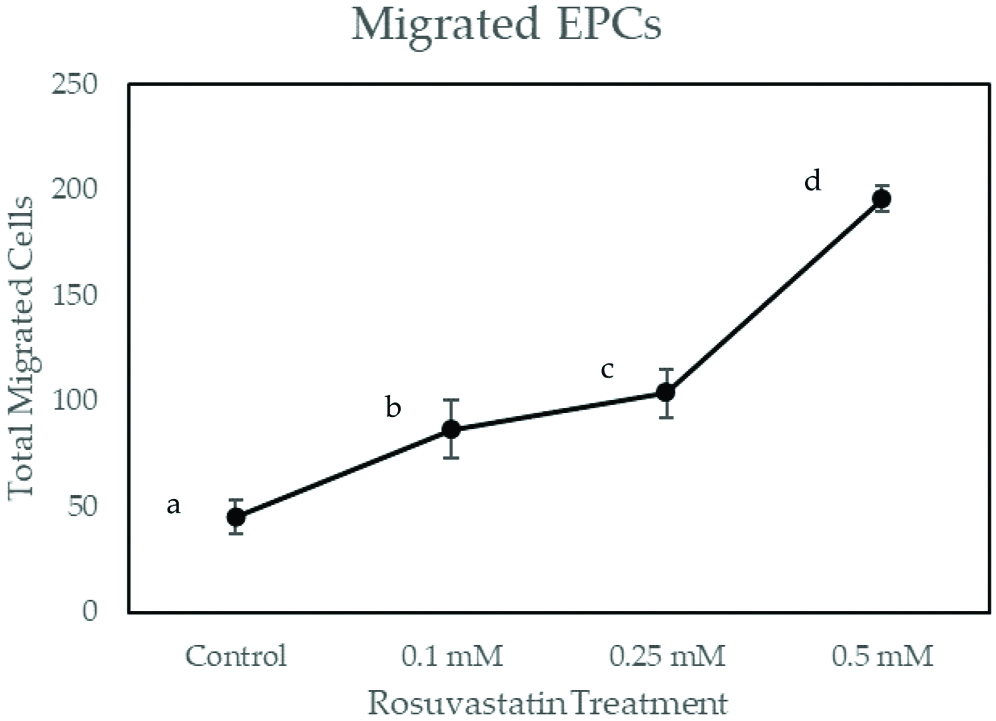
As shown in [Table/Fig-4], migrated EPCs on rosuvastatin dose 0.1 mM, 0.25 mM and 0.5 mM have had significant differences with the control group (p<0.001). EPCs migrations were increased in dose-dependent manner, which were 86,625.00±13,930.63 for 0.1 mM rosuvastatin, 103,500.00±11, 250 for 0.25 mM rosuvastatin and 19,5750.00±5809.475 for 0.5 mM rosuvastatin. Pearson’s correlation showed significant and very strong correlation between rosuvastatin treatment with the number of migrated EPCs (r=0.810; p≤0.001). Linear regression test showed r-square of 0.656. This suggested that rosuvastatin treatment is responsible for 65.6% of EPCs migration.
Total Migrated EPCs on Increasing Dose of Rosuvastatin Treatment.
EPCs were exposed to increasing concentrations of rosuvastatin for 48 hours. Migrated EPCs was determined via Boyden Chamber Assay. Total migrated cells are expressed as mean±SE (n=4). Different annotation (a,b,c,d) denounce significant difference in ANOVA test (p<0.001)
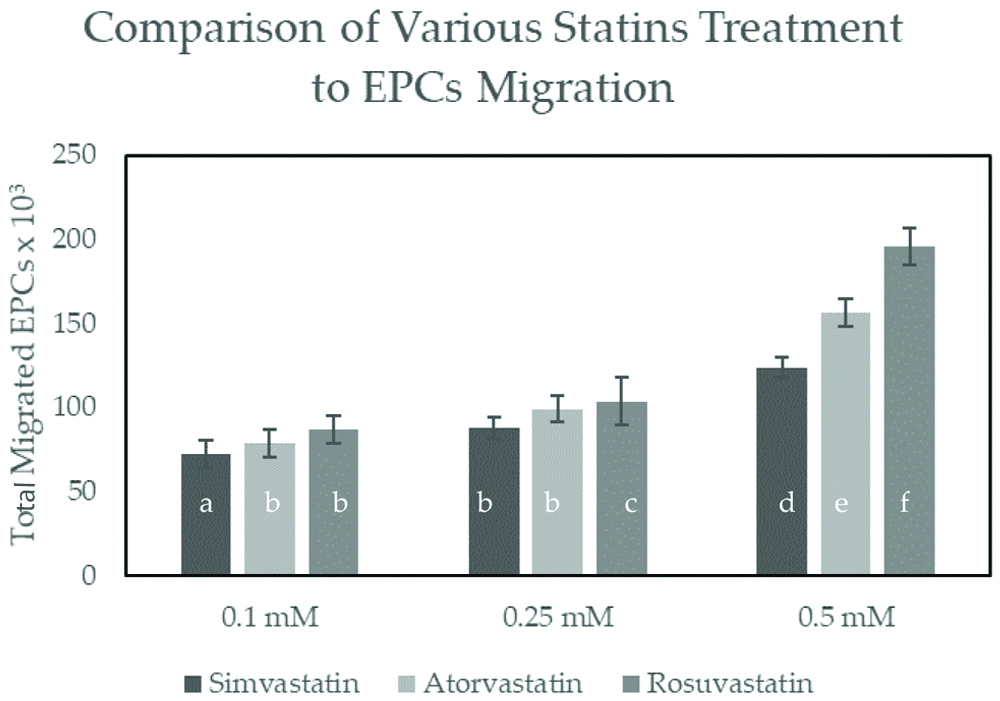
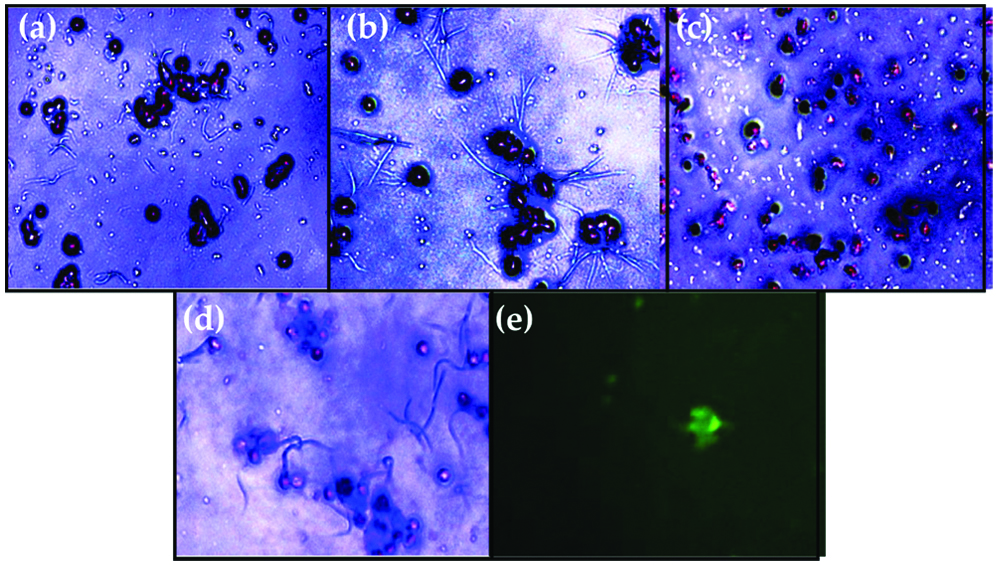
As shown in [Table/Fig-5], there were significant differences of migrated cells in rosuvastatin treatment compared to simvastatin and atorvastatin at the same dose (p<0.05). At the highest dose (0.5 mM), rosuvastatin have significantly higher number of migrated EPCs (195,750.00±5,809.48) compared to simvastatin (123,750.00±9,367.50, p≤0.001) and atorvastatin (156,375.00±12,392.03, p≤0.001). Microscopic view of the culture can be seen on [Table/Fig-6a-f], showing the EPCs cell that has been migrated from Boyden Chamber Assay and stained with trypan blue. These findings suggested that rosuvastatin is superior in increasing EPCs migration compared to simvastatin and atorvastatin at the highest dose (0.5 mM).
Comparison between simvastatin, atorvastatin and rosuvastatin at 0.1 mM, 0.25 mM and 0.5 mM dose on Total Migrated EPCs.
Total migrated cells are expressed as mean±SE (n=4). Different annotation (a,b,c,d,e,f) denounce significant difference in ANOVA test (p<0.05)
Light-inverted microscopic view of migrated EPCs stained with trypan blue after 48 hours of treatment with: (a); Simvastatin dose 0.5 mM; (b) Atorvastatin dose 0.5 mM; (c) Rosuvastatin dose 0.5 mM; (d) Control; (e) EPCs confirmation using FITC-labeled anti-human CD34 observation with immunofluorescence microscope.
Discussion
Impaired EPCs function can be improved with statin treatments. In this research, treatment with simvastatin, atorvastatin, and rosuvastatin were able to increase EPCs migration in dose-dependent manner. This finding was consistent with the previous finding which showed that simvastatin treatment on impaired EPCs culture from diabetic rats improved its migration capabilities in a dose-dependent manner at dose 0.01, 0.1, 1 mmol/L [18]. For other statins, usage of various doses on EPCs treatment was rarely used [19]. However, treatment with atorvastatin and rosuvastatin at dose 2.5 μM, 5 μM and 10 μM on EPCs culture in presence of SDF-1 have been shown to have dose-dependent manner effect on the vascular tube forming capacity in vitro [20], while vascular tube forming capacity is correlated with EPCs migration capability [21]. Hence, this suggests an indirect proof of dose-dependent effect of atorvastatin and rosuvastatin on EPCs migration capability.
While this research showed dose-dependent effect of simvastatin, atorvastatin and rosuvastatin on EPCs migration in-vitro, in-vivo or clinical trial might show a different result. Atorvastatin treatment on CHD patients showed increased migrated EPCs in the circulation, but no difference of migrated EPCs on treatment dose 10 mg and 40 mg [22]. The previous meta-analysis also suggested that statins increased EPCs migration in clinical trials. However, only a few studies compared statins at the different dose, making it unclear whether a dose-dependent relationship exists [19].
In this research, the highest regression percentage was shown on simvastatin treatment (92.9%) compared to atorvastatin (75.5%) and rosuvastatin (65.6%). Suggesting that simvastatin will have the lowest unexplained variation of EPCs migration as the outcome (7.1%) assuming all other variables are controlled. While the mechanism underlying the different effect of various type of statins on EPC migration still needs to be determined. Previous research showed that simvastatin treatment has a high regression percentage with the NOX2 (NADPH Oxidase 2) reduction (76%) [20]. Similarly, atorvastatin and rosuvastatin also proved to have NOX2 inhibition effect [21]. NOX2 level was proven to have a negative correlation with EPCs migration and adhesion [22], suggesting that antioxidant pathway which involves NOX2 might be dominant pathway that explain the effect of simvastatin, atorvastatin and rosuvastatin treatment on EPCs migration. NOX2 inhibition on statin treatment has been proven to be correlated with increased NO availability [23]. While, NO is essential for EPCs proliferation and migration [24,25].
In terms of efficiency, the highest migration was observed on rosuvastatin (195,750.00±5,809.48) compared to simvastatin (123,750.00±9,367.497, p≤0.001) and atorvastatin (156,375.00±12,392.03) at the highest dose (0.5 mM). While authors suggest the antioxidant pathway might play a dominant role in the EPCs migration effect for the statin treatment, the present authors also observed similar efficiency comparison between statins on its antioxidant effect. Atorvastatin treatment has a significantly higher reduction of oxidative stress compared to simvastatin on patients with coronary heart disease [26]. Rosuvastatin treatment also has a significantly higher reduction in Rho/Rho kinase (ROCK) activity which is activated from oxidative stress compared to atorvastatin [27]. However, at the low dose (0.1 mM) no significant difference on EPCs migration between atorvastatin and rosuvastatin.
Limitation
The present evidence suggested that simvastatin, atorvastatin, and rosuvastatin have a beneficial effect in EPCs migration, there is a lack of consensus for EPCs definition (Hibbert). Although, this research confirms EPCs through CD34 immunofluorescence, which is the most commonly-used surface marker to identify EPCs [19]; other studies suggested using CD34+/KDR+ with either CD45-, CD45dim through flow cytometry to accurately confirm EPCs count [19]. This suggests possibilities of other cells other than EPCs also being counted.
Conclusion
Simvastatin, atorvastatin and rosuvastatin treatment improve EPCs migration from CAD patients in dose-dependent manner. High dose rosuvastatin is superior compared to atorvastatin and simvastatin to increase the EPCs migration.