Psoriasis is a common immune-mediated disease involving skin and joints of genetically predisposed individuals that typically follows a relapsing and remitting course [1]. In approximation, psoriasis affects 2-3% of people worldwide, and its prevalence in Egypt ranges from 0.19-3% [2].
Psoriasis has many clinical types including; psoriasis vulgaris (chronic plaque psoriasis), guttate psoriasis, erythrodermic psoriasis, palmoplantar psoriasis, psoriatic arthritis, inverse psoriasis and pustular psoriasis {generalised pustular psoriasis (von Zumbusch type), impetigo herpetiformis and localised pustular psoriasis (palmoplantar pustular psoriasis and acrodermatitis continua)}. The classic lesion of psoriasis is a sharply demarcated, red papule covered by silvery-white scales [3]. Regarding psoriasis treatment, it can be categorised into five groups that include topical preparations (e.g., topical steroids, salicylic acid and calcipotriol), traditional systemic agents (e.g., methotrexate tablets and vials), phototherapy (such as narrow band ultraviolet B), biologic therapy (e.g., adalimumab) and experimental approaches [4].
The aetiology of psoriasis is unknown; it is a multifactorial disorder with numerous key constituents including genetic susceptibility (e.g., Tumour Necrosis Factor-alpha (TNF-α) gene polymorphism) [5], environmental triggering agents (e.g., stress, weather and sunlight) and skin barrier distraction (e.g., trauma) in addition to immune dysfunction including innate and acquired immune dysfunction [3]. External triggers including infections e.g., Staphylococcus aureus, Malassezia, Candida albicans and HIV can activate an initial episode of psoriasis in those individuals who already have a genetic predisposition [4]. Also, HCV was reported among infections that may trigger psoriasis [6].
The development of various extra-hepatic manifestations by HCV likely involves autoimmune mechanisms (immune-related extrahepatic manifestations) [10], while others seem to be driven by chronic inflammation (inflammatory-related extrahepatic manifestations) [9]. The immune-related extrahepatic manifestations are reinforced by the fact that HCV is lymphotropic and patients with HCV have increased autoantibodies [11]. Interaction between HCV and lymphocytes directly modifies B and T cell function resulting in increased polyclonal activation and expansion of B cell producing immunoglobulins. Also, decreased CD4+CD25+FoxP3+regulatory T cells were reported which could account for the increase in the peripheral auto-reactive B cells [9]. Additionally, evaluating the impact of antiviral therapy on HCV-associated dermatologic disorders may clarify the mechanisms behind these disorders. Simultaneous improvement in HCV viremia and a dermatologic disorder would also support direct viropathic mechanisms [7,12].
The association between hepatitis C and psoriasis showed conflicting results. Some authors have found no increase in the rate of HCV infection in patients with psoriasis or psoriatic arthropathy [13], while others have found increased prevalence of HCV infection in patients with psoriasis [8,14,15] and those having psoriatic arthropathy [16].
To examine the relationship between psoriasis and HCV, previous researchers investigated serum HCV antibody levels [15,17,18] and/or plasma HCV RNA concentration [8] using ELISA and real time PCR, respectively. Additionally, HCV mRNA in the tissue of psoriasis patients was detected in psoriatic lesion in one patient having psoriasis vulgaris and another one having pustular psoriasis [19]. Till date; there has been no study that investigated HCV protein in skin of psoriasis patients.
Therefore, the aim of this study was to evaluate the possible role of HCV in aetiopathogenesis of psoriasis through immunohistochemical assessment of HCV protein in involved skin of HCV positive patients having psoriasis vulgaris compared to HCV positive non-psoriatic patients and controls, in addition to study the relation between this expression with clinical and histological aspects of psoriasis in those patients.
Materials and Methods
This study was a case-control study. It was conducted in Departments of Dermatology, Andrology and Sexually Transmitted Diseases (STDs), Internal Medicine and Pathology, Faculty of Medicine, Menoufia University, spanning the period from May 2016 to February 2017. In the current study, the sample size was 60 subjects. They were 20 psoriasis vulgaris patients having HCV infection (group I) and their age was 32-65 years, 20 age (31-58 years) and sex matched non-psoriatic HCV positive cases (group II) and 20 (36-57 years) healthy volunteers (group III). The investigated individuals were selected according to inclusion and exclusion criteria. Patients with psoriasis vulgaris from both sexes not receiving any topical or systemic treatment for psoriasis one month before joining the study were included. However, those having systemic or cutaneous immuno-inflammatory diseases and/or uncontrolled infections were excluded from this study. Additionally, patients who received anti-HCV drugs within the last three months were also excluded.
A written consent form was obtained from every participant before the study initiation. The study was approved by the Committee of Human Rights in Research at Menoufia University having IEC number of 1104/12/4/2016.
Methods
Complete history was elicited from the individuals including: personal history (name; age; sex; residence; occupation; special habits of medical importance), present history (onset, course and disease duration), past history (medical and drug history) and family history (of psoriasis and any other diseases). Dermatological examination was done. Diagnosis of psoriasis was based on its clinical presentation (red papules and plaques covered by silver white scales that showed Auspitz sign) and confirmed by histological findings of the skin biopsies. Assessment of course of psoriasis [20] and PASI score to evaluate the severity of psoriasis were also performed.
PASI is the most commonly used tool for assessment of psoriasis severity. To calculate PASI score for each of studied patient, the body was divided into four sections: 1) head (h) (10% of a person’s skin); 2) upper limb (u) (20%); 3) trunk (t) (30%); and 4) lower limb (l) (40%). For each section, the percent of area of skin involved was estimated and transformed into a grade from 0 to 6; 0=0% of involved area; 1=<10% of involved area, 2=10-29% of involved area, 3=30-49% of involved area, 4=50-69% of involved area, 5=70-89% of involved area and 6=90-100% of involved area.
Within each area (A), the severity was estimated by three clinical signs; erythema (E) (redness), desquamation (D) (scaling) and induration (I) (thickness). These severity parameters were measured on a scale of 0 to 4; 0=none, 1=slight, 2=moderate, 3=severe and 4=very severe.
The sum of these three severity parameters was then calculated for each skin section, and was multiplied by the area score for that area and by weight of respective section (0.1 for head, 0.2 for upper limb, 0.3 for trunk and 0.4 for lower limb). The final formula for PASI score is:
PASI=0.1 (Eh+Ih+Dh) Ah+0.2 (Eu+Iu+Du) Au+0.3 (Et+It+Dt) At+0.4 (El+Il+Dl) Al [21].
PASI ranged from 0 (no disease) to 72 (maximal). A PASI score <7 denoted mild psoriasis, from 7 to 12 denoted moderate and more than 12 meant severe form of psoriasis [22].
For all participants, ELISA was done to estimate HCV antibodies. No HCV antibodies were detected in control volunteers i.e. group II and III of the patient groups (ELISA positive cases), PCR was performed to confirm the diagnosis of HCV infection.
Three millimeters Punch biopsies were taken under 2% lignocaine local anesthesia from involved skin of psoriasis patients and from matched site of control groups. Specimens were fixed in 10% formalin solution, and submitted to routine tissue processing to be embedded in paraffin blocks.
From each specimen, sections of 4 μm thickness were cut on routine slides for haematoxylin and eosin staining to assess the pathological changes in psoriatic patients including acanthosis, hyperkeratosis, parakeratosis, capillary proliferation, microabcesses and dermal inflammatory infiltrate [23].
For immune staining, sections were cut on Poly L Lysine coated slides. The method used was streptavidin-biotin amplified system. The primary antibody used was monoclonal Anti-HCV antibody. It was a purified mouse monoclonal (Cat. # GTX40671) raised against hepatitis C NS5A antigen. It is received as concentrated form from GeneTex International Corporation (Global) 6F-2,NO.89, Dongmei Rd., East Dist., Hsinchu City 300 Taiwan, R.O.C. Tel: 886-3-6208988, Fax: 886-3-6208989.
Immunohistochemically, HCV protein expression is confirmed by nuclear staining in the epidermis and/or the dermis according to supplier’s datasheet. In the epidermis, the percentage of the positive cell were assessed subjectively at 200x magnification field and intensity of the stain was subjectively graded as mild, moderate or strong. Using the evaluated percentage of the HCV protein positive cells and its assessed intensity, histo-score (H score) was calculated for all studied specimens by this equation;
H score=1×percentage of mildly stained cells+2×percentage moderately stained cells+3×percentage of strongly stained cells [24].
H score is a histological score that depends on:
Percentage of positive cells.
Intensity of colour in these positive cells: evaluated subjectively as mild, moderate or strong.
Cutaneous load: is the amount of the viral particles in the skin
It was known for its H score. H score depends on percentage of positive cells (cells showing HCV protein positivity) and its intensity that reflect amount of HCV particles.
H score is indicative of cutaneous HCV viral load.
Statistical Analysis
Data were collected, tabulated and statistically analysed using a personal computer with Statistical Package for Social Science (SPSS) version 15 program (SPSS Inc., Chicago, U.S). Qualitative data was expressed as: number and percentage. Quantitative data was expressed as: arithmetic mean (x2), Standard Deviation (SD), percentage (%) and median. For comparing qualitative variables Chi-square test (χ2-test) was used, Mann-Whitney U test (U test) was used in comparing two non-parametric quantitative variables. Kruskal-Wallis test (K test) was used for comparing non-parametric three or more quantitative variables. The p≤0.05 was considered statistically significant.
Results
Demographic and clinico-pathologic data of studied subjects: Demographic data of studied individuals and clinical criteria of investigated psoriasis patients as well as its histopathological changes are demonstrated in [Table/Fig-1]. There were no significant differences among the three studied groups {HCV positive psoriasis (group I), HCV positive patients without psoriasis (group II) and controls (group III)} regarding their age and sex (p>0.05 for all) [Table/Fig-1].
Clinico-pathological data of studied subjects.
| Studied group n=60 | Test of significance | p-value |
|---|
| HCV with psoriasis n=20 | HCV without psoriasis n=20 | Control n=20 |
|---|
| N | % | N | % | N | % |
|---|
| Sex |
| Male | 12 | 60 | 15 | 75 | 14 | 70 | FXT1.108 | 0.632 |
| Female | 8 | 40 | 5 | 25 | 6 | 30 |
| Age (years) |
| Mean±SD | 51.1±13.6 | 43.04±12.4 | 47.5±9.2 | F test1.41 | 0.253 |
| Range | 32-65 | 31-58 | 36-57 |
| Median | 45 | 52 | 29 |
| Psoriasis duration/years |
| Mean±SD | 8.9±4.3 | ---- | ---- | ---- | ---- |
| Range | 2-19 |
| Median | 9 |
| Age of psoriasis onset (years) |
| Mean±SD | 38.5±7.4 | ---- | ---- | ---- | ---- |
| Range | 28-53 |
| Median | 36 |
| PASI score |
| Mean±SD | 11.1±2.6 | ---- | ---- | ---- | ---- |
| Range | 3-14.3 |
| Median | 12.2 |
| Disease progression |
| Stable | 7 | 35 | ---- | ---- | ---- | ---- |
| Progressive | 13 | 65 |
| Disease severity |
| Mild | 2 | 10 | ---- | ---- | ---- | ---- |
| Moderate | 7 | 35 |
| Severe | 11 | 55 |
| Family history |
| Yes | 6 | 30 | ---- | ---- | ---- | ---- |
| No | 14 | 70 |
| Hyperkeratosis |
| Mild | 9 | 45 | ---- | ---- | ---- | ---- |
| Moderate | 9 | 45 |
| Severe | 2 | 10 |
| Parakeratosis |
| Negative | 5 | 25 | ---- | ---- | ---- | ---- |
| Positive | 15 | 75 |
| Munro micro abscess |
| Negative | 16 | 80 | ---- | ---- | ---- | ---- |
| Positive | 4 | 20 |
| Capillary proliferation |
| Negative | 2 | 10 | ---- | ---- | ---- | ---- |
| Mild | 4 | 20 |
| Moderate | 10 | 50 |
| Severe | 4 | 20 |
| Epidermal hyperplasia |
| Mild | 5 | 25 | ---- | ---- | ---- | ---- |
| Moderate | 6 | 30 |
| Severe | 9 | 45 |
| Inflammation |
| Negative | 2 | 10 | ---- | ---- | ---- | ---- |
| Mild | 6 | 30 |
| Moderate | 8 | 40 |
| Severe | 4 | 20 |
No: Number; FXT: Fisher’s-exact test; PASI: Psoriasis area severity index; F test: ANOVA test
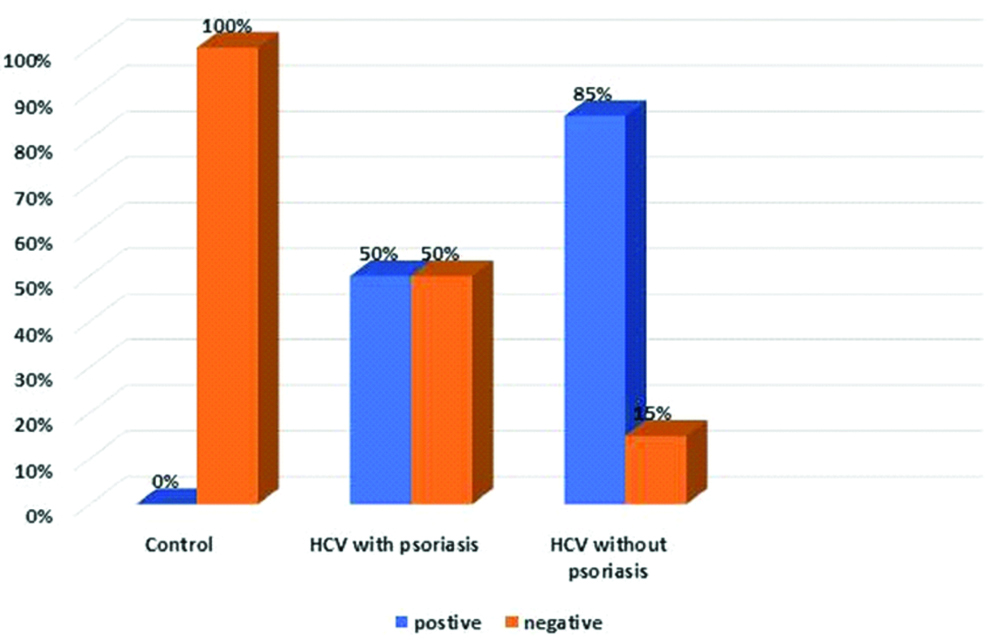
HCV protein immunohistochemical expression: All of the examined biopsies from healthy individuals (group III) revealed complete absence of HCV protein expression [Table/Fig-2]. While, 85% of HCV positive non-psoriatic patients (group II) revealed positive expression of HCV protein immunoreactivity with mild intensity in 64.7% of them [Table/Fig-3], and half (50%) of HCV positive psoriatic patients (group I) demonstrated positive expression of HCV protein immunoreactivity with moderate and strong intensity in 60% of them [Table/Fig-4]. Comparison of HCV protein expression in the three studied groups showed statistically significant difference (p=0.001) [Table/Fig-5].
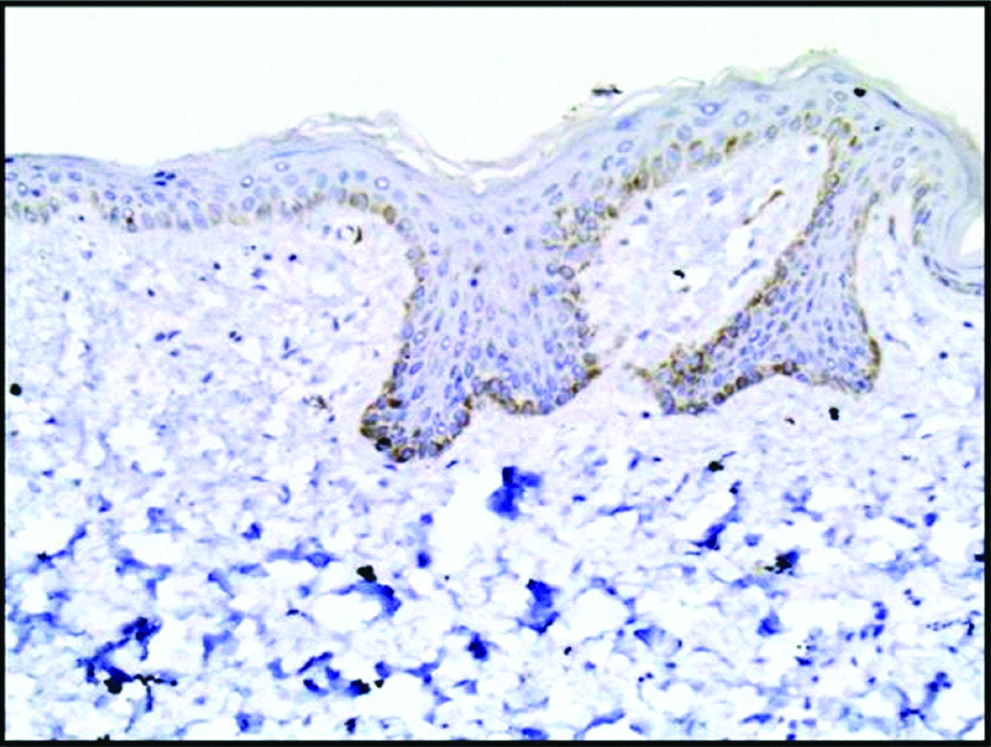
Normal skin section from control subjects (group III) showed negative expression of HCV protein (immunoperoxidase, 200X).
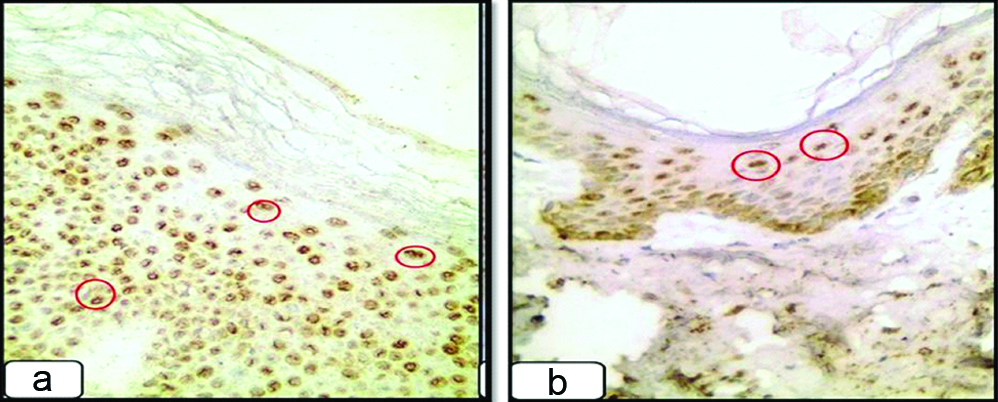
HCV positive non-psoriatic section (group II) showed mild (a) and moderate (b) expression of HCV protein (red circles) in the epidermis {immunoperoxidase, (a) 200X; (b) 100X}.
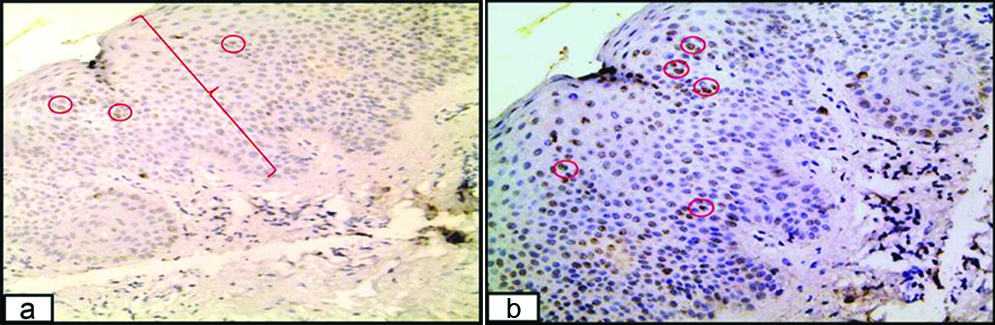
HCV positive psoriasis section (group I) demonstrated moderate (a) and strong (b) expression of HCV protein (red circles) in the epidermis (immunoperoxidase 200X).
Comparison between studied groups regarding HCV protein expression.
Although positivity of HCV protein immunoreactivity was significantly lower in psoriasis than non-psoriasis HCV positive cases (p=0.02), its percentage and H-score mean values were significantly higher in psoriasis patients than non-psoriasis cases (p=0.044; p=0.03), respectively [Table/Fig-6].
Comparison between HCV positive psoriatic and non-psoriatic patients regarding HCV protein immunoreactivity.
| Studied group n=40 | Test of significance | p-value |
|---|
| HCV with psoriasis n=20 | HCV without psoriasis n=20 |
|---|
| N | % | N | % |
|---|
| Expression |
| Positive | 10 | 50 | 17 | 85 | χ2 | 0.02* |
| Negative | 10 | 50 | 3 | 15 | 5.58 |
| In case of positive expression |
| Percent |
| Mean±SD | 39.5±22.9 | 17.9 ±14.7 | | 0.044* |
| Range | 10-80 | 5-60 | U |
| Median | 40 | 10 | 2.10 |
| H score |
| Mean±SD | 75.5±60.4 | 33.8±43.3 | | 0.03* |
| Range | 10-180 | 5-180 | U |
| Median | 62.5 | 20 | 2.15 |
| Intensity |
| Mild | 4 | 40 | 11 | 64.7 | | 0.40 |
| Moderate | 5 | 50 | 5 | 29.4 | FXT |
| Strong | 1 | 10 | 1 | 5.9 | 1.48 |
| Inflammatory cell |
| Positive | 7 | 70 | 14 | 82.4 | FXT | 0.66 |
| Negative | 3 | 30 | 3 | 17.6 | 0.232 |
FXT: Fisher’s exact test; χ2: Chi-square test; U: Man-Whiteny test; *Significant
HCV protein positive versus HCV protein negative psoriasis patients: The studied psoriasis patients (n=20) were divided according to HCV protein immunoreactivity into two groups; HCV protein positive (n=10) and negative (n=10) groups. Compared to HCV protein negative immunoreactive psoriasis cases, HCV protein positive psoriasis patients had significantly severe form of psoriasis (p=0.032), but insignificant differences regarding other clinical criteria and histopathological parameters [Table/Fig-7].
Comparison between HCV protein positive and negative psoriasis patients regarding their clinico-pathological data.
| Clinico-pathological data | Expression in HCV with psoriasis | Test of significance | p-value |
|---|
| Positive n=10 | Negative n=10 |
|---|
| N | % | N | % |
|---|
| Sex |
| Male | 7 | 70 | 5 | 50 | FXT0.833 | 0.650 |
| Female | 3 | 30 | 5 | 50 |
| Age (years) |
| Mean±SD | 46.8±10.5 | 48.2±8.3 | t0.330 | 0.74 |
| Range | 44 | 48.5 |
| Median | 33-65 | 32-57 |
| Psoriasis duration/years |
| Mean±SD | 9.6±4.1 | 8.2±4.7 | U0.989 | 0.322 |
| Range | 4-15 | 2-19 |
| Median | 11 | 7.5 |
| Age of psoriasis onset (years) |
| Mean±SD | 38.6±8.5 | 38.5±6.6 | t0.029 | 0.97 |
| Range | 29-53 | 28-51 |
| Median | 36.5 | 37.5 |
| PASI score |
| Mean±SD | 11.2±3.3 | 11.1±1.8 | t0.114 | 0.910 |
| Range | 3-14.2 | 9.3-14.3 |
| Median | 12.4 | 10 |
| Family history of psoriasis |
| Positive | 4 | 40 | 2 | 20 | FXT0.952 | 0.62 |
| Negative | 6 | 60 | 8 | 80 |
| Disease progression |
| Stable | 7 | 70 | 6 | 60 | FXT0.220 | 1.00 |
| Progressive | 3 | 30 | 4 | 40 |
| Disease severity |
| Mild | 2 | 20 | 0 | 0 | FXT6.39 | 0.032* |
| Moderate | 1 | 10 | 6 | 60 |
| Severe | 7 | 70 | 4 | 40 |
| Hyperkeratosis |
| Mild | 4 | 40 | 5 | 50 | FXT2.72 | 0.33 |
| Moderate | 6 | 60 | 3 | 30 |
| Severe | 0 | 0 | 2 | 20 |
| Parakeratosis |
| Negative | 4 | 40 | 1 | 10 | FXT2.4 | 0.30 |
| Positive | 6 | 60 | 9 | 90 |
| Munro micro abscess |
| Negative | 9 | 90 | 7 | 70 | FXT1.25 | 0.26 |
| Positive | 1 | 10 | 3 | 30 |
| Capillary proliferation |
| Negative | 0 | 0 | 2 | 20 | FXT4.1 | 0.23 |
| Mild | 2 | 20 | 2 | 20 |
| Moderate | 7 | 70 | 3 | 30 |
| Severe | 1 | 10 | 3 | 30 |
| Epidermal hyperplasia |
| Mild | 2 | 20 | 3 | 30 | FXT3.6 | 0.19 |
| Moderate | 5 | 50 | 1 | 10 |
| Severe | 3 | 30 | 6 | 60 |
| Inflammation |
| Negative | 0 | 0 | 2 | 20 | FXT4.5 | 0.209 |
| Mild | 3 | 30 | 3 | 30 |
| Moderate | 6 | 60 | 2 | 20 |
| Severe | 1 | 10 | 3 | 30 |
U: Mann-Whiteny test; t: Student t-test; FXT: Fisher’s-exact test; *S: Significant; H and E: Haematoxylin and Eosin
Comparison between high and low H-score in HCV protein positive psoriasis patients regarding their clinico-pathological data: To study the relationship between H-score of HCV protein expression and studied data in HCV protein positive psoriasis patients (n=10), they were subdivided into two groups according to H-score median value (62.5); group A (n=4) having H-score <62.5 and group B (n=6) having H-score ≥62.5. Compared to group A, psoriasis patients in group B had longer disease duration (11.2±5.2 vs 6.16±3.37) (p=0.05). However, the relations between H-score and other clinico-pathological parameters could not reach level of significance (p>0.05) [Table/Fig-8].
Relationship between H score of HCV protein immune-staining with clinico-pathological criteria of HCV protein positive psoriasis patients.
| Clinico-pathological data | Hscore in HCV with psoriasis | Test of significance | p-value |
|---|
| Low H-score≤62.5 n=4 | High H-score≥62.5 n=6 |
|---|
| N | % | N | % |
|---|
| Sex |
| Male | 2 | 50 | 5 | 83.3 | FXT1.27 | 0.33 |
| Female | 2 | 50 | 1 | 16.7 |
| Age (years) |
| Mean±SD | 52±7.7 | 43.3±11.2 | t1.33 | 0.22 |
| Range | 50 | 41 |
| Median | 6 | 9 |
| Psoriasis duration/years |
| Mean±SD | 6.16±3.37 | 11.2±5.2 | U1.93 | 0.05* |
| Range | 2-12 | 8-19 |
| Median | 6 | 9 |
| Age of psoriasis onset/years |
| Mean±SD | 40.7±8.6 | 37.1±8.8 | t0.631 | 0.54 |
| Range | 33-53 | 29-53 |
| Median | 38 | 35 |
| PASI score |
| Mean±SD | 10.8±1.71 | 11.2±2.2 | t0.499 | 0.631 |
| Range | 9.3-13.4 | 9.6-14.3 |
| Median | 10 | 10.9 |
| Family history of psoriasis |
| Positive | 3 | 75 | 1 | 16.7 | FXT3.40 | 0.19NS |
| Negative | 1 | 25 | 5 | 83.3 |
| Disease progression |
| Stable | 4 | 100 | 3 | 50 | FXT2.85 | 0.20 |
| Progressive | 0 | 0 | 3 | 50 |
| Disease severity |
| Moderate | 2 | 50 | 2 | 33.3 | FXT0.27 | 1.00 |
| Severe | 2 | 50 | 4 | 66.7 |
| Hyperkeratosis |
| Mild | 3 | 75 | 1 | 16.7 | FXT3.40 | 0.19 |
| Moderate | 1 | 25 | 5 | 83.3 |
| Parakeratosis |
| Negative | 3 | 75 | 1 | 16.7 | FXT3.40 | 0.19 |
| Positive | 1 | 25 | 5 | 83.3 |
| Munro micro abscess | Negative | 4 | 100 | 1 | 16.7 | FXT1.27 | 0.50 |
| Positive | 0 | 0 | 5 | 83.3 |
| Capillary proliferation |
| Negative | 0 | 0 | 0 | 0 | FXT0.77 | 0.100 |
| Mild | 1 | 25 | 1 | 16.7 |
| Moderate | 3 | 75 | 4 | 66.7 |
| Severe | 0 | 0 | 1 | 16.7 |
| Epidermal hyperplasia |
| Mild | 2 | 50 | 0 | 30 | FXT3.3 | 0.19 |
| Moderate | 1 | 25 | 4 | 66.7 |
| Severe | 1 | 25 | 2 | 33.3 |
| Inflammation |
| Negative | 0 | 0 | 0 | 0 | FXT1.66 | 0.71 |
| Mild | 2 | 50 | 1 | 16.7 |
| Moderate | 2 | 50 | 4 | 66.7 |
| Severe | 0 | 0 | 1 | 16.7 |
U: Mann-Whiteny test; t: Student t-test; FXT: Fisher’s-exact test; *S: Significant; H and E: Haematoxylin and Eosin
Discussion
The relationship between hepatitis C and psoriasis is still a matter of discussion. Therefore, this study was designed to shed light on the hypothesised role of HCV in psoriasis pathogenesis, through evaluation of HCV protein {non-structural protein 5A (NS5A)} tissue expression in involved psoriatic skin compared to controls, and to evaluate this expression to clinico-pathologic aspects of psoriasis in those patients.
There is a relatively high prevalence of HCV infection among psoriatic patients. Also, most of the patients had HCV infection before development of psoriasis. Taken together, these two observations intensely suggest that HCV infection is an inducer for psoriasis. HCV is a chronic systemic inflammatory disorder that mediated overproduction of TNF-α, which induces psoriasis in those having a certain genetic and/or environmental tendency [25]. Supporting this hypothesis, treatment of psoriasis with TNF-α antagonists without worsening of HCV was reported [26].
In HCV, NS5A is a proline-rich and zinc-binding hydrophilic phosphoprotein. It is originated from a large protein precursor (polyprotein) which is translated from the HCV genome. Then, it undergoes post translation processing by non-structural protein 3 viral protease [27]. NS5A has an important role in HCV RNA replication. Additionally, it is a key moderator in regulating host cell response and activity upon HCV infection [28].
In the current study, HCV protein immunoreactivity was observed in half of the studied psoriatic cases demonstrating moderate and strong intensity in 60% of them. However, in HCV positive non-psoriatic patients, HCV protein expression was significantly higher than those of psoriasis patients (85%), but was mostly of mild intensity. Therefore, we could not support the suggestion that presence of HCV infection participates in occurrence of psoriasis.
Although positivity of HCV protein immunoreactivity was significantly lower in psoriatic than non-psoriatic HCV positive cases, its percentage and H-score mean values were significantly higher in psoriatic patients. Therefore, it was suggested that not the presence of HCV infection, but its cutaneous load may have an active role in pathogenesis of psoriasis.
The demonstrated HCV protein (NS5A), the key player in HCV RNA replication [29], in the psoriatic plaques in the current study is supported by an earlier work done by Yamamoto T et al., [19]. The authors described HCV mRNA in involved skin in psoriasis vulgaris (n=1) and pustular psoriasis (n=1) patients using PCR.
Development of an autoimmune disorder, such as psoriasis, is the risk associated with HCV that could be a result of exacerbating reactions against the organism’s own tissues and/ or an elevated risk of the development of de novo autoimmune responses, triggered frequently by Interferon-Alpha (IFN-α) [30]. Induction/exacerbation of psoriasis after IFN therapy of HCV patients was observed. IFN-α was considered to be an essential cytokine produced by pathogenic dermal plasmacytoid dendritic cells of psoriatic skin [31,32]. Therefore, administration of IFN-α may substitute for this pathway, inducing psoriasis in HCV infected patients [30].
Moreover, systemic inflammation and Tumour Necrosis Factor Alpha (TNF-α) have an important role in pathogenesis of psoriasis [33]. HCV is associated with chronic inflammation and aberrant immune response; therefore, HCV may independently trigger or exacerbate psoriasis. Moreover, TNF-α is a common mediator for both diseases [25,34].
Furthermore, Chun K et al., confirmed recently the immunological association between HCV and psoriasis [35]. They observed that the inflammatory genes responsible for psoriasis development (Cathelcidin, Toll like receptor 9 and an interferon gamma) showed increased expression in HCV positive psoriatic patients.
In this study, most of the psoriasis patients (90%) were presented by moderate and severe forms of psoriasis. Moreover, HCV protein immunoreactivity was significantly associated with severe psoriasis. Confirming these results, Gabr SA et al., on their study on 90 psoriatic patients with positive HCV antibodies showed significant increase in PASI score mean values (p<0.001) suggesting that HCV infection may contribute to the severity of psoriasis [18]. Also, Taha EA et al., found positive correlation between viral load and PASI score [36].
In group I HCV positive psoriasis patients, high H-score of HCV protein expression was significantly associated with long disease duration. Supporting this finding, Mohamed AE et al., reported positive correlation between psoriasis duration and viral load [8]. Moreover, it was observed that the expression of HCV protein was associated with male gender, younger group and positive family history. However, these associations could not reach level of significance that could be attributed to small sample size in this study.
Limitation
The main limitation of the current work was small sample size. Therefore, further large scale studies to validate our results and to assess the role of HCV protein in other types of psoriasis. Additionally, in depth studies to investigate the molecular pathway by which HCV could participate in psoriasis pathogenesis were also recommended.
Conclusion
From the result of the current study, it may be concluded that; not the presence of HCV infection, but its cutaneous load may have an active role in pathogenesis of psoriasis. This cutaneous load was positively associated with disease duration. Moreover, HCV infection doesn’t only increase psoriasis severity, but also may have a role in its different clinical aspects.
No: Number; FXT: Fisher’s-exact test; PASI: Psoriasis area severity index; F test: ANOVA test
FXT: Fisher’s exact test; χ2: Chi-square test; U: Man-Whiteny test; *Significant
U: Mann-Whiteny test; t: Student t-test; FXT: Fisher’s-exact test; *S: Significant; H and E: Haematoxylin and Eosin
U: Mann-Whiteny test; t: Student t-test; FXT: Fisher’s-exact test; *S: Significant; H and E: Haematoxylin and Eosin