Isolated Renal Zygomycosis, A Rare and Lethal Cause of Massive Renal Infarction- Report of Two Cases
Salapathi Shanmugam1, B Rajeshwari2, Sadiya Niamath3, Mitra Ghosh4
1 Junior Consultant, Department of Histopathology, Apollo Speciality Hospitals, Vanagaram, Chennai, Tamil Nadu, India.
2 Junior Consultant, Department of Histopathology, Apollo Speciality Hospitals, Vanagaram, Chennai, Tamil Nadu, India.
3 Senior Consultant, Department of Histopathology, Apollo Speciality Hospitals, Vanagaram, Chennai, Tamil Nadu, India.
4 Senior Consultant and Head, Department of Histopathology, Apollo Speciality Hospitals, Vanagaram, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Salapathi Shanmugam, Junior Consultant, Department of Laboratory Services, Apollo Speciality Hospitals, Vanagaram-600095, Chennai, Tamil Nadu, India.
E-mail: salapsdr@gmail.com
Zygomycosis (Mucormycosis) is an infection caused by a class Zygomycetes fungi. The fungal organisms classified as Mucorales order cause a spectrum of predominantly angio-invasive disease in immunosuppressed patients. While renal involvement is not so uncommon in disseminated infections, isolated renal involvement is rare. Here we report two cases of isolated renal mucormycosis who presented with abdominal pain. Radiological evaluation showed evidence of pyelonephritis. On histopathological examination complete renal infarction with thrombosed vessels caused by invasive fungal hyphae morphologically suggestive of Mucorales. Renal zygomycosis should be suspected in immunocompromised patients and in patients with abdominal trauma or following surgery with acute abdomen and persistent fever, despite negative urine and blood culture. We present the cases here for their rarity and clinical importance.
Angio-invasion, Mucorales, Mucormycosis
Case Reports
Case 1
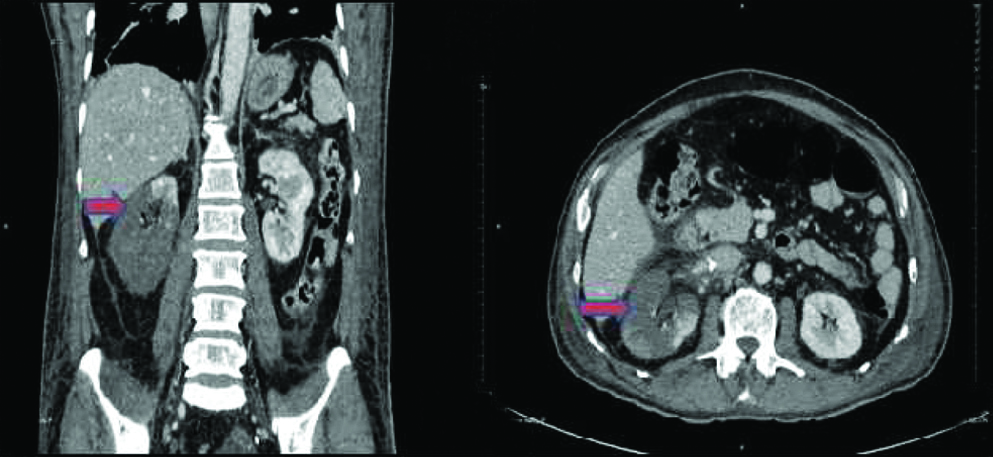
A 65-year-old male, known case of type 2 diabetes presented with sudden onset high grade intermittent fever, bilateral renal angle pain for 2 weeks, treated with antibiotics in an outside hospital. Despite treatment, his symptoms worsened and admitted to our hospital. On examination patient was conscious, oriented and febrile, with abdominal distention and bilateral renal angle tenderness. Clinical examination of cardiovascular (CVS) and respiratory system didn’t show any abnormality. There were no other systemic signs or symptoms. Blood investigations showed neutrophilic leukocytosis with a total count of 27,130/cumm and blood urea of 106 mg/dL and serum creatinine of 3.7 mg/dL. Random blood sugar level was 256 mg/dL with glycosylated haemoglobin (HbA1c) level of 14%. Urine routine examination showed 2 to 4 pus cells/High Power Field (HPF) with no bacteria. Blood, urine and ascitic fluid cultures didn’t show any growth. Chest X-ray showed bilateral apical pleural thickening/scarring with no lung parenchymal lesion. Echocardiogram showed no pericardial effusion or vegetations. Computed Tomography (CT) of abdomen done at our hospital showed evidence of bilateral pyelonephritis with complete infarction of right kidney [Table/Fig-1]. He underwent emergency laparotomy and right nephrectomy and kidney was sent for histopathological examination.
CT abdomen of case 1 shows diffuse infarction of right kidney with minimal sparing of upper pole (arrow) and diffuse perinephric fat stranding.
Case 2
A 58-year-old male developed breathing difficulty, acute abdominal pain and fever and clinically diagnosed to have acute appendicitis in an outside hospital for which he underwent laparoscopic appendicectomy. Post-procedure his symptoms worsened with haematuria and acute kidney injury and referred to our hospital. On evaluation, the patient was conscious, oriented and febrile, tachypnoeic with abdominal distention. Patient was not a known case of type 2 diabetes or any other systemic illness. Respiratory system examination showed reduced breath sounds in the bilateral basal regions of lung. CVS examination didn’t show any abnormality. There are no other systemic signs or symptoms. Blood investigations showed neutrophilic leukocytosis with a total count of 19,270/cumm and blood urea 153 mg/dL and serum creatinine was 3.1 mg/dL. Random blood sugar level was 147 mg/dL with HbA1c level of 6.3%. Urine routine examination showed plenty of red blood cells, with 2 to 4 pus cells/HPF with no bacteria. Blood and urine cultures didn’t show any growth. Chest X-ray showed bilateral minimal pleural effusion with no lung parenchymal lesion. Echocardiogram showed no pericardial effusion or vegetations. Patient was treated with intravenous antibiotics and other supportive therapy. Patient’s general condition remained same. CT abdomen showed diffusely bulky right kidney with features suggestive of renal infarction. He underwent emergency right radical nephrectomy and specimen was received for histopathological examination.
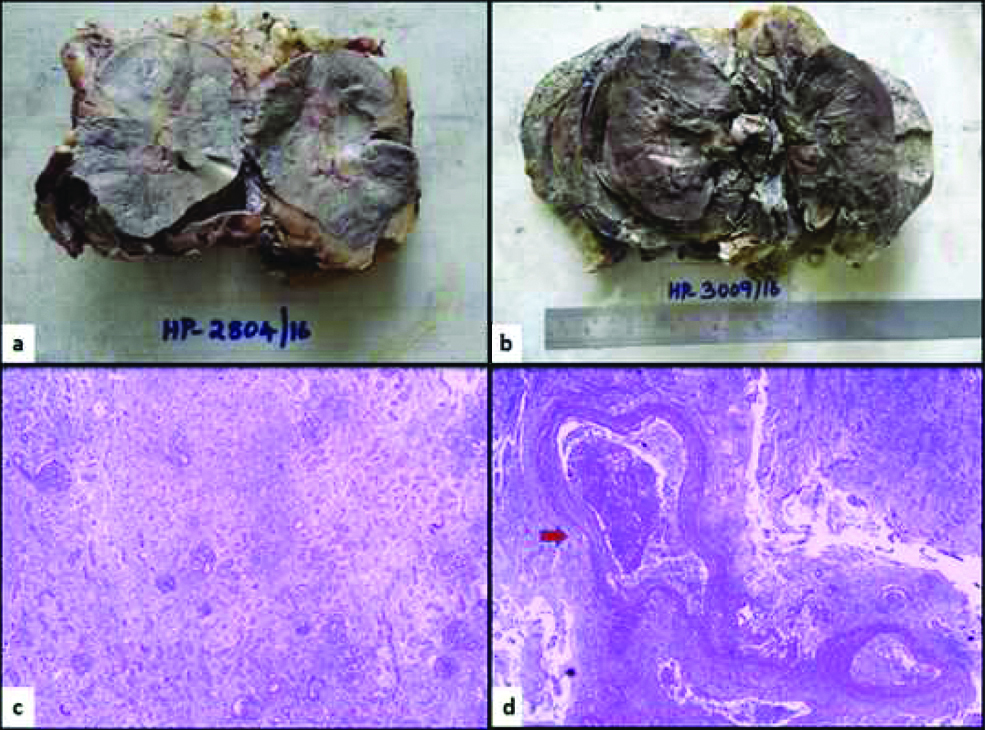
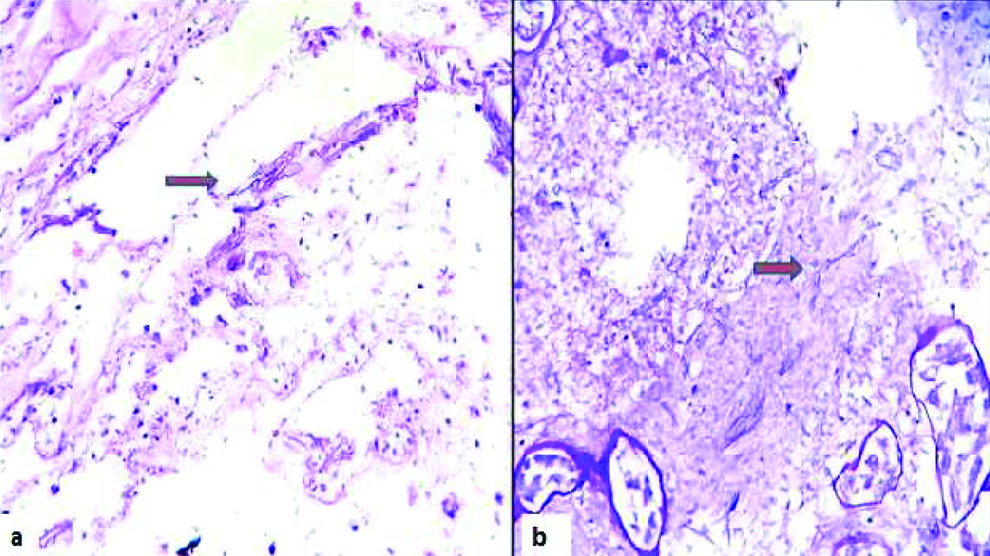
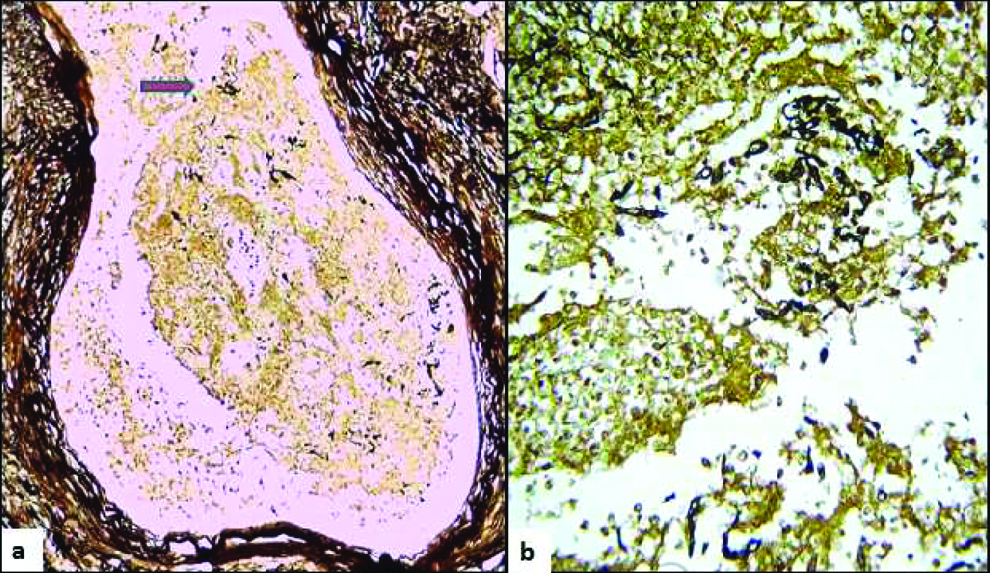
In both the cases, the gross examination of nephrectomy specimens showed grey brown to black discolouration suggestive of extensive renal infarction with adherent perirenal fat. Few thrombosed vessels were noted in the hilum. Microscopic examination showed extensive renal infarction with only focal viable areas [Table/Fig-2]. Necrotic debris containing invasive fungal organism with broad, irregular aseptate/pauciseptate hyphae and right angle branching morphologically suggestive of Mucorales family seen, which were confirmed by fungal stains Gomari methenamine silver (GMS), Periodic acid Schiff (PAS). There was extensive angio-invasion by fungal hyphae with thrombosed vessels [Table/Fig-3,4]. Perirenal fat and ureters also showed infarcted areas, with ulceration and invasive fungal hyphae. Since the diagnosis of Mucormycosis was not suspected preoperatively, fungal cultures were not done in both the cases. In both our cases there was no evidence of any other site involvement clinically and radiologically. Antifungal treatment with intravenous amphotericin B was started in both the cases following the histopathological diagnosis. In case 1 the patient became oligoanuric with severe metabolic acidosis and hypotension due to septic shock and died on 4th postoperative day. In case 2 the patient initially improved following amphotericin B therapy and hemodialysis, but later developed anuria, altered liver parameters and coagulopathy and eventually died on 13th postoperative day.
Nephrectomy specimens with grey brown discoloration of infarcted kidney, and adherent perirenal fat (a and b). Infarcted renal parenchyma with outlines of tubules and few preserved glomeruli (c), Thrombosed blood vessels (arrow) in the renal hilum, (d), H&E stain (40x).
(a) Ribbon like irregular fungal hyphae (arrow) in infarcted renal parenchyma, H&E stain (400x), b) highlighted by PAS stain, 400x.
(a) Scanner view of thrombosed vessel with fungal hyphae in the luminal thrombus, (b) High power shows pauciseptate ribbon like fungal hyphae in the lumen. GMS stain, 400x.
Discussion
Mucormycosis is an infection caused by a class Zygomycetes fungi [1]. Mucorales genera are ubiquitous in the environment and can be found in soil and decomposing matter. Their spores are easily airborne, and inhalation or direct inoculation into skin can cause rhinocerebral, pulmonary and cutaneous infections [2]. Any of these primary manifestations can give rise to disseminated disease. Mortality due to disseminated disease is extremely high but varies depending on the associated risk factor and clinical presentation [3]. Of the Mucorales, Rhizopus is the genus most frequently causing human disease, while Mucor species cause disease in fewer than 20% of cases [2]. Rhinocerebral and pulmonary involvement is more common, and renal involvement can occur in disseminated disease, however isolated renal involvement is rare [4,5]. Mucorales are able to quickly disseminate because of their angio-invasive property, hence rapid diagnosis and treatment is necessary [6,7]. The renal involvement by angio-invasive fungi such as Aspergillus and Mucor has been found to be associated with increased morbidity and mortality [3,8]. Mucormycosis has been known to occur in the otherwise healthy individuals also [9,10]. Healthcare-associated mucormycosis are also reported following invasive medical procedures and following surgery [11]. In one of these cases the patient was in immunocompromised state because of uncontrolled type 2 Diabetes. In other case, the patient probably contracted infection following abdominal surgery for acute appendicitis. In renal mucormycosis, the affected kidney fungi may be involved in a focal or diffuse manner [8]. They are often enlarged in size and show infarction with thrombosed hilar vessels [12]. The major morphological differentiation is between Mucorales genera and other fungi that produce non-pigmented hyphae in tissue, including Aspergillus species., other hyaline septated moulds (such as Fusarium), and Candida species [2]. The presence of narrow fungal hyphae with abundant septation and acute-angle branching should suggest the diagnosis of Aspergillus species or another hyaline septate moulds, while yeasts with pseudohyphae should suggest Candida species. Poor staining of hyphae with GMS often suggest Mucormycosis, hence it is always better to ask both PAS and GMS stains when suspecting fungal infection in histopathology [2,13]. Though histopathology with fungal stains can help to rule out other species, fungal culture is the gold standard for definitive diagnosis and to rule out mixed infections. Histopathological identification of fungus elements will rule out culture contamination and will also demonstrate if there is angio-invasion [2,13]. Sometimes the hyphae may be degenerated, and many of the fungal characteristics may not be appreciated in the specimen. Other ancillary diagnostic tools helpful to identify Mucorales are Immunohistochemistry (IHC), Polymerase Chain Reaction (PCR) or in situ hybridization [2,14]. As mentioned earlier, culture of the tissue specimen is indispensable for organism-specific diagnosis. Although serologic tests have been attempted, they are not recommended [2].
Isolated renal mucormycosis is very rare. Mostly single case reports are reported so far. Ranjan P et al., reported a case of isolated mucormycosis of kidney presenting as acute renal failure in a non--diabetic adult male [8]. Gupta V et al., reported a case of renal and splenic mucormycosis in a healthy adult presented with fever and flank pain [10]. In a large retrospective study conducted by Gupta KL et al., in a tertiary centre, 45 cases of mucormycosis were reported over a period of 12 years. In those, 18 cases were with renal involvement and only nine of them were isolated renal mucormycosis without any systemic involvement. Thirteen of the renal mucormycosis cases in their study were diagnosed after autopsy only and majority of them had infarcted kidney [6].
Conclusion
Renal zygomycosis should be considered in immunocompromised patients and in patients following abdominal trauma or surgery who have persistent flank pain and fever, but sterile urine and blood cultures. The fungal order of Mucorales are known to quickly disseminate because of their angio-invasive property, hence rapid diagnosis with initiation of appropriate surgical and antifungal treatment is important. Nephrectomy and appropriate antifungal treatment are the standard therapy. Fungal culture of tissue specimen is gold standard for diagnosis. However histopathological examination is important to demonstrate angioinvasion, and also will rule out culture contamination. Isolated renal involvement by Mucorales is not so common and the case is presented here for its rarity and for its clinical significance.
[1]. Pak J, Tucci VT, Vincent AL, Sandin RL, Greene JN, Mucormycosis in immunochallenged patientsJ Emerg Trauma Shock 2008 1(2):106-13.10.4103/0974-2700.4220319561989 [Google Scholar] [CrossRef] [PubMed]
[2]. Guarner J, Brandt ME, Histopathologic diagnosis of fungal infections in the 21st centuryClin Microbiol Rev 2011 24(2):247-80.10.1128/CMR.00053-1021482725 [Google Scholar] [CrossRef] [PubMed]
[3]. Gupta KL, Fungal infections and the kidneyIndian J Nephrol 2001 11:147-54. [Google Scholar]
[4]. Chakrabarti A, Das A, Sharma A, Panda N, Das S, Gupta KL, Ten years’ experience in zygomycosis at a tertiary care centre in IndiaJ Infect 2001 42(4):261-66.10.1053/jinf.2001.083111545569 [Google Scholar] [CrossRef] [PubMed]
[5]. Lussier N, Laverdiere M, Weiss K, Poirier L, Schick E, Primary renal mucormycosisUrology 1998 52(5):900-03.10.1016/S0090-4295(98)00287-8 [Google Scholar] [CrossRef]
[6]. Gupta KL, Joshi K, Sud K, Kohli HS, Jha V, Radotra BD, Renal zygomycosis: an under-diagnosed cause of acute renal failureNephrol Dial Transplant 1999 :2720-25.10.1093/ndt/14.11.272010534520 [Google Scholar] [CrossRef] [PubMed]
[7]. Marak RSK, Misra R, Ansari MS, Dixit A, Poornima , Prasad KN, Successful medical management of renal zygomycosis: A summary of two cases and a review of the Indian literatureMedical Mycology 2010 48(8):1088-95.10.3109/1369378100375347720367111 [Google Scholar] [CrossRef] [PubMed]
[8]. Ranjan P, Chipde SS, Vashistha S, Kumari N, Kapoor R, Reno-invasive fungal infection presenting as acute renal failure: importance of renal biopsy for early diagnosisSaudi J Kidney Dis Transpl 2014 25(6):1282-84.10.4103/1319-2442.14426825394451 [Google Scholar] [CrossRef] [PubMed]
[9]. Verma R, Vij M, Agrawal V, Jain M, Renal mucormycosis in immunocompetent patients: Report of three casesBasic and Applied Pathology 2011 4(2):66-70.10.1111/j.1755-9294.2011.01102.x [Google Scholar] [CrossRef]
[10]. Gupta V, Singh SK, Kakkar N, Jain S, Kalra N, Sakia UN, Splenic and renal mucormycosis in a healthy host: successful management by aggressive treatmentTropical Gastroenterology 2010 31(1):57-58. [Google Scholar]
[11]. Rammaert B, Lanternier F, Zahar JR, Dannaoui E, Bougnoux ME, Lecuit M, Healthcare-associated mucormycosisClin Infect Dis 2012 54(1):44-54.10.1093/cid/cir86722247444 [Google Scholar] [CrossRef] [PubMed]
[12]. Vesa J, Bielsa O, Arango O, Lladó C, Gelabert A, Massive renal infarction due to mucormycosis in an AIDS patientInfection 1992 20(4):234-36.10.1007/BF020330681521891 [Google Scholar] [CrossRef] [PubMed]
[13]. Frater JL, Hall GS, Procop GW, Histologic features of zygomycosis: Emphasis on perineural invasion and fungal morphologyArch Pathol Lab Med 2001 125(3):375-78. [Google Scholar]
[14]. Sunagawa K, Ishige T, Kusumi Y, Asano M, Nisihikawa E, Kato M, Renal abscess involving mucormycosis by immunohistochemical detection in a patient with acute lymphocytic leukemia: A case report and literature reviewJpn J Infect Dis 2013 66(4):345-47.10.7883/yoken.66.34523883851 [Google Scholar] [CrossRef] [PubMed]