Validation of Hemochroma PLUS: A Point of Care Testing Device for Haemoglobin Estimation
Shyamsundar J Raithatha1, Mustafa F Ranapurwala2, Simmy Lahori3, Chandrashekhar N Bopche4, Ajay Gajanan Phatak5
1 Associate Professor, Department of Community Medicine, Pramukhswami Medical College, Karamsad, Gujarat, India.
2 Associate Professor, Department of Pathology, Pramukhswami Medical College, Karamsad, Gujarat, India.
3 Intern, Department of Community Medicine, Pramukhswami Medical College, Karamsad, Gujarat, India.
4 Executive, Department of Extension Programmes, Charutar Arogya Mandal, Karamsad, Anand, Gujarat, India.
5 Manager, Department of Central Research Services, Charutar Arogya Mandal, Karamsad, Anand, Gujarat, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mustafa F Ranapurwala, Pramukhswami Medical College and Shree Krishna Hospital, Karamsad, Gujarat, India.
E-mail: dr.mustafa.r@gmail.com
Introduction
Anaemia is a major public health challenge in India. Advent of Point of Care (POC) testing devices for haemoglobin estimation may be useful in the field settings, albeit after proper validation of these devices.
Aim
To assess the validity of Hemochroma PLUS in haemoglobin estimation by comparing it with an automated laboratory analyser in rural Indian context.
Materials and Methods
A cross-sectional study was conducted at a tertiary care rural teaching hospital during November-December 2017. Adults visiting the out-patient laboratory collection unit for a haemogram test were included in the study. Venous blood samples were collected by trained phlebotomists and tested using autoanalyser in the laboratory. Capillary blood samples were collected by a trained study coordinator using a lancet (Ez-life) and tested on a Hemochroma PLUS device. Haemoglobin measurement by both methods was compared using Bland-Altman analysis. The analysis was performed using STATA (14.2).
Results
A total of 100 (53 females and 47 males) adults participated in the study. Mean (SD) age of the participants was 44.62 (16.92) years. Bland-Altman analysis revealed that the mean difference (95% confidence limits) was 0.3 (-2.0, 2.7) gm/dL. Hemochroma PLUS was found to have sensitivity of 84.90% and specificity of 82.98% in identifying anaemia.
Conclusion
Hemochroma PLUS showed good agreement with the cell counter in current study. Further studies may be undertaken in different study populations and study settings. Hemochroma PLUS, has a potential as a POC tool for community-based anaemia screening and evaluation of anaemia control programmes.
Anaemia, Bedside testing, Cell counter, Method comparison
Introduction
Haemoglobin estimation is probably the most commonly performed biochemical test. At the population level, it is used for mapping and management of haemoglobin disorders like sickle cell and thalassaemia as well as anaemia [1]. In hospital settings, it is used for variety of purposes including transfusion decision in perioperative phase [2], identifying anaemia in emergency room patients [3], checking eligibility of blood donors, etc. [4]. As the haemoglobin estimation is used in clinical decision making, it is expected that the test used is rapid, economical and reasonably accurate.
The world witnessed a technological leap in last few decades that enabled evolution of diagnostic testing. Manual methods of haemoglobin estimation like Sahli Technique or haemoglobin colour scale that lacked in accuracy are replaced by auto analysers. Although accurate, auto analysers lack in portability and are expensive [5]. These facts provided an impetus to develop POC technologies that can provide reasonably accurate results and are portable, as stressed in ‘Grand Challenges in Global Health’ [6].
Anaemia, one of the biggest public health problems faced by India; mostly affects children and women. As per the 4th National Family Health Survey (2015-2016), more than 50% of the population in the 5-59 month age group was found to be anaemic in Anand district of Gujarat (India) with a similar prevalence in women in reproductive age group (15-49 years) as well [7]. Correction of anaemia provides significant socio-economic benefits to the country in addition to the health benefits.
POC technologies can provide convenient, economical, rapid and reasonably accurate haemoglobin estimation for screening population at large in identification and management of anaemia. However, it is important to immaculately validate the new technology before adopting it [8]. HemoCue® appears to be most popular POC device for haemoglobin estimation and its validation is extensively researched [3,9-11]. The latest POC technology viz., Hemochroma PLUS has better storage capacity over HemoCue® and claims to have low maintenance-repair cost. However, Hemochroma PLUS is not validated adequately [4,12] and when validated, used an improper analysis process of Pearson’s correlation coefficient and regression [12].
This study was designed to assess the validity of Hemochroma PLUS in haemoglobin estimation by comparing it with an automated laboratory haematology analyser in a rural Indian context.
Materials and Methods
A cross-sectional study was conducted at Shree Krishna Hospital, a tertiary care rural teaching hospital during November-December 2017 which was approved by institutional ethics committee. A total of 100 adults (aged more than 18 years) visiting the out-patient laboratory collection unit of Shree Krishna Hospital, Karamsad, Anand (Gujarat) for a haemogram test were included in the study. Voluntary written informed consent was obtained from all participants after explaining the purpose of the study and procedure. The venous blood samples were collected by trained phlebotomists and sent to the Central Diagnostic Laboratory (CDL) of the hospital for haemoglobin testing using an automated cell counter (Sysmex XN550). The CDL is an NABL (National Accreditation Board for Laboratories) accredited laboratory and follows all the quality improvement protocols as prescribed by NABL [13]. Capillary blood samples were collected by a trained study coordinator using a lancet (Ez-life) and tested on a Hemochroma PLUS (BODITECH MED INC., KOREA) device [Table/Fig-1]. The capillary blood was collected on a micro cuvette and the micro cuvette was placed in the device which provided results in less than 60 seconds using a dual wavelength photometry. Age, gender and laboratory numbers of each participant were collected in addition to the haemoglobin readings by Hemochroma PLUS and the cell counter.

Sample Size
Measuring agreement is an estimation problem and hence larger sample size will provide stable estimates of mean difference as well as the lower and upper confidence limits. However, there is a trade-off between larger sample size and gain in precision. Martin J Bland who invented this simple method for measuring agreement between two methods, suggested a minimum sample size of 100 for such studies [8]. Considering the feasibility, a sample size of 100 was considered for this study.
Statistical Analysis
Descriptive statistics {mean (SD), frequency (%)} were used to portray the characteristics of the study participants. Bland-Altman analysis was used to assess agreement between the two methods of haemoglobin estimation viz., Cell counter method and Hemochroma PLUS. Anaemia status as identified by both methods was compared. The analysis was performed using STATA 14.2 (StataCorp LP).
Results
Out of the 100 adults {53 Females, 47 Males} contacted for the study, all agreed to participate in the study. The mean (SD) (Median: Range) age of the participants was 44.62 (16.92) (44.50: 15, 78) years. The mean (SD) (Median: Range) haemoglobin by cell counter method was 11.98 (2.26) (11.90: 4.80, 17.70) whereas mean (SD) (Median: Range) haemoglobin by Hemochroma PLUS was 11.66 (2.42) (11.50: 5.60, 17.70) gm/dL.
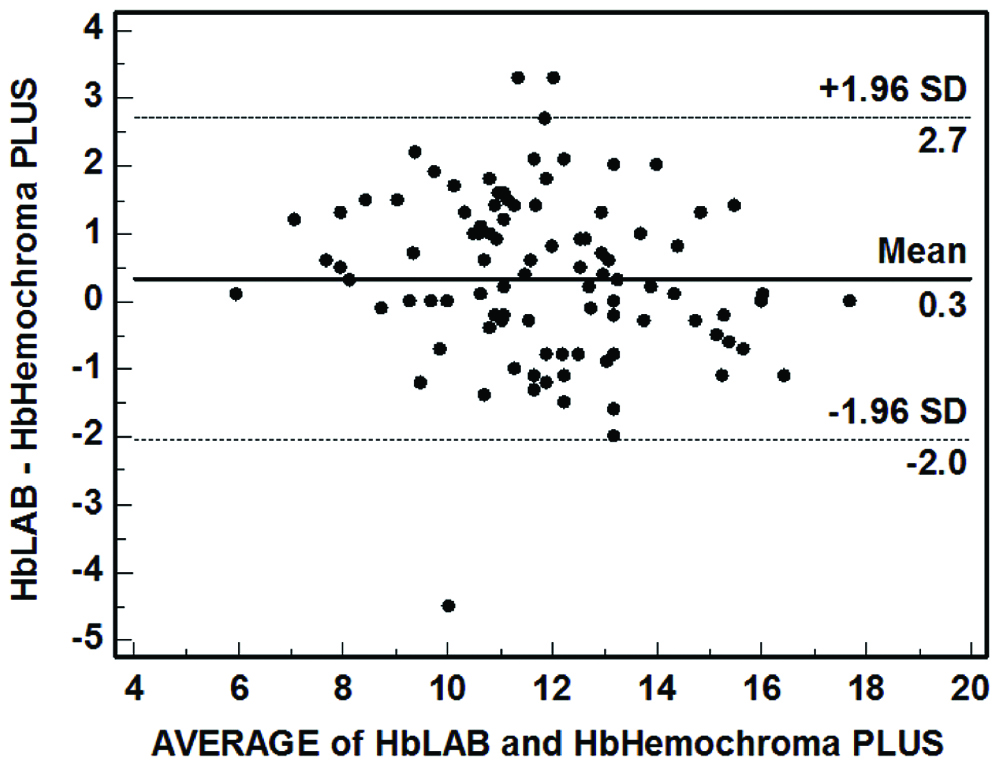
Bland-Altman plot revealed that the differences were reasonably normally distributed without any specific upward/downward trend. The mean difference (95% confidence limits) was 0.3 (-2.0, 2.7) gm/dL [Table/Fig-2]. Further exploration of data revealed that the Hemochroma PLUS was able to correctly identify anaemia status in 83 cases. It overestimated anaemia status in nine cases and underestimated it in eight cases [Table/Fig-3]. After collapsing the categories as Anaemia and No Anaemia, Hemochroma PLUS was found to have sensitivity of 84.90% and specificity of 82.98%.
Bland-Altman plot assessing agreement between Hemochroma PLUS and cell counter (n=100).
Identification of anaemia status by Hemochroma PLUS as compared with lab results (n=100).
| Anaemia status as per Hemochroma PLUS | Anaemia status as per Cell Counter (Lab) reports |
|---|
| No Anaemia (>12 gm%) | Mild/moderate Anaemia (7-12 gm%) | Severe Anaemia (<7 gm%) | Total |
|---|
| No Anaemia (>12 gm%) | 39 | 8 | 0 | 47 |
| Mild/moderate Anaemia (7-12 gm%) | 8 | 42 | 0 | 50 |
| Severe Anaemia (<7 gm%) | 0 | 1 | 2 | 3 |
| Total | 47 | 51 | 2 | 100 |
*The categories were collapsed as Anaemia and No Anaemia to find sensitivity and specificity of Hemochroma PLUS
Discussion
Haemoglobin values obtained from Hemochroma PLUS and laboratory analyser agreed satisfactorily with each other in classifying the severity of anaemia and mean difference in the measurements by the two techniques was quite low. This finding is important in deciding the utility of Hemochroma PLUS as a POC test for haemoglobin estimation.
Hemochroma PLUS is a recently introduced POC device for haemoglobin estimation and has the advantage of better storage and less maintenance cost over HemoCue. It was found very easy to use and the data transfer was smooth. Hemochroma PLUS works on dual wavelength photo-absorption method. The degree of light absorption is measured with a spectrophotometer. The fixed optical distance between the Hemochroma PLUS microcuvette walls permit photometric determination of haemoglobin in undiluted blood sample [14]. In a way, it uses a sensor based technology albeit invasive. One study on validation of Hemochroma PLUS which compared it with a cell counter (Coulter AcT) used correlation and regression analysis [12]. However, correlation is not an appropriate method for assessing agreement [8]. Another study that compared Hemochroma PLUS with automated blood analyser BC 3000 Plus reported similar mean difference but tight confidence limits compared to present study [4].
Many attempts were made in the past to check the validity of HemoCue with respect to auto analysers and found it reasonably accurate with variable mean difference and confidence limits [2,3,9-11]. HemoCue works on similar principle used in Hemochroma PLUS. A recent study performed systematic review and meta-analysis of method comparison studies involving comparison of non-invasive POC Haemoglobin estimation devices (Masimoto Radical-7™ or Pronto-7™) as well as POC HemoCue absorption spectrometers (B-Haemoglobin or 201+) with laboratory measurements using a cell counter [15]. This study revealed that the HemoCue devices have better agreement than non-invasive POC technologies. The pooled mean difference was less than the present study and the confidence limits were tighter.
The focal point of the study was to check the validity of Hemochroma PLUS. The reliability of the device needs to be confirmed before adopting it to practice. Further the proportion of participants with severe anaemia (Hb <7) was quite low. Thus, the findings need to be validated in population with severe anaemia, healthy population, paediatric patients and patients with haemoglobin disorders etc.
POC technologies can do wonders in healthcare management. For example, screening of neonates for critical congenital heart defects using pulse oximetry (SPO2) has demonstrated the utility of such technology in early detection that improves surgical outcome and reduces morbidity, though the incidence of critical congenital heart defects is low [16]. The prevalence of anaemia in Indian context is far greater and it leads to adverse maternal and neonatal outcomes. On the contrary, early detection and management of anaemia proves to improve the maternal as well as neonatal outcome [17].
Government set ups are still using manual methods for haemoglobin estimation (mainly Sahli technique) that is prone to erroneous results. It is probably time to move from manual methods to more accurate invasive POC technologies and finally to non-invasive technologies. However, the environmental conditions should be considered along with training of POC device users and universal SOPs [18].
Limitation
The study was conducted in winter months (November-December, 2017). The operation temperature of Hemochroma PLUS is 15~35 degree Celsius. The device might malfunction in summer months when the temperature rises above 40°C across India.
Conclusion
Keeping in mind the affordability and access to laboratory facilities in Indian setting coupled with simplicity and satisfactory accuracy of Hemochroma PLUS, it has a potential as a POC tool in classifying severity of anaemia and treatment decision making as well as for community-based anaemia screening and evaluation of anaemia control programmes.
*The categories were collapsed as Anaemia and No Anaemia to find sensitivity and specificity of Hemochroma PLUS
[1]. Modell B, Darlison M, Global epidemiology of haemoglobin disorders and derived service indicatorsBulletin of the World Health Organization 2008 86:480-87.10.2471/BLT.06.03667318568278 [Google Scholar] [CrossRef] [PubMed]
[2]. Srinivasan N, Kasturba M, Intra-operative point of care haemoglobin estimation: a comparison of three methodsSri Lankan Journal of Anaesthesiology 2010 18(1):15-19.10.4038/slja.v18i1.1555 [Google Scholar] [CrossRef]
[3]. Zatloukal J, Pouska J, Kletecka J, Pradl R, Benes J, Comparison of the accuracy of Haemoglobin point of care testing using HemoCue and GEM Premier 3000 with automated hematology analyzer in emergency roomJournal of Clinical Monitoring and Computing 2016 30(6):949-56.10.1007/s10877-015-9799-z26507548 [Google Scholar] [CrossRef] [PubMed]
[4]. Anukul N, Sombatmai R, Leetrakool N, Somphan P, Evaluation of capillary Haemoglobin measurement from portable Haemoglobinometers in blood donor screeningJ Hematol Transfus Med 2018 28:121-29. [Google Scholar]
[5]. Srivastava T, Negandhi H, Neogi SB, Sharma J, Saxena R, Methods for Haemoglobin estimation: A review of “what works”J Hematol Transfus 2014 2(3):1028 [Google Scholar]
[6]. Varmus H, Klausner R, Zerhouni E, Acharya T, Daar AS, Singer PA, Grand challenges in global healthScience 2003 302(5644):398-99.10.1126/science.109176914563993 [Google Scholar] [CrossRef] [PubMed]
[7]. National Family Health Survey 4(2015-16), District Fact Sheet-Anand, Gujarat. Available from: http://rchiips.org/nfhs/nfhs3.shtml. Accessed January 30, 2019 [Google Scholar]
[8]. Phatak AG, Nimbalkar SM, Method Comparison (Agreement) Studies: Myths and RationaleJ Clin Diagn Res 2017 11(1):JI01-03.10.7860/JCDR/2017/23897.931428273982 [Google Scholar] [CrossRef] [PubMed]
[9]. Sanchis-Gomar F, Cortell-Ballester J, Pareja-Galeano H, Banfi G, Lippi G, Haemoglobin point-of-care testing: The HemoCue systemJ Lab Autom 2013 18(3):198-205.10.1177/221106821245756022961038 [Google Scholar] [CrossRef] [PubMed]
[10]. Shah N, Osea EA, Martinez GJ, Accuracy of noninvasive Haemoglobin and invasive point-of-care Haemoglobin testing compared with a laboratory analyzerInt J Lab Hematol 2014 36(1):56-61.10.1111/ijlh.1211823809685 [Google Scholar] [CrossRef] [PubMed]
[11]. Hiscock R, Simmons SW, Carstensen B, Gurrin LC, Comparison of Massimo Pronto-7 and HemoCueHb 201+ with laboratory haemoglobin estimation: A clinical studyAnaesth Intensive Care 2014 42(5):60810.1177/0310057X140420051025233174 [Google Scholar] [CrossRef] [PubMed]
[12]. Wanyama FM, Sekadde-Kigondu C, Maturi P, Analytical evaluation of hemochroma POC haemoglobin readerJ Med Diagn Meth 2016 5(219):210.4172/2168-9784.1000219 [Google Scholar] [CrossRef]
[13]. National Accredition Board for Laboratories (NABL). Specific Criteria For Accreditation Of Medical Laboratories. 2018. Available from: https://www.nabl-india.org/nabl/index.php?c=publicaccredationdoc&m=index&docType=both&Itemid=199 Accessed May 13, 2018 [Google Scholar]
[14]. Department of Health and Human Services, Food and Drug Administration, USA. Section 8:510(k) Summary, hemochroma PLUS System. 2017:5. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf16/K163465.pdf Accessed 13 March, 2019 [Google Scholar]
[15]. Hiscock R, Kumar D, Simmons SW, Systematic review and meta-analysis of method comparison studies of Masimo pulse co-oximeters (Radical-7™ or Pronto-7™) and HemoCue® absorption spectrometers (B-Haemoglobin or 201+) with laboratory haemoglobin estimationAnaesth Intensive Care 2015 43(3):341-50.10.1177/0310057X150430031025943608 [Google Scholar] [CrossRef] [PubMed]
[16]. Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK, Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysisThe Lancet 2012 379(9835):2459-64.10.1016/S0140-6736(12)60107-X [Google Scholar] [CrossRef]
[17]. Christian P, Mullany LC, Hurley KM, Katz J, Black RE, Nutrition and maternal, neonatal, and child healthSemin Perinatol 2015 39(5):361-72.10.1053/j.semperi.2015.06.00926166560 [Google Scholar] [CrossRef] [PubMed]
[18]. Yadav K, Olivia MJ, Ahamed F, Mandal M, Kant S, Use of Point of Care Testing (POCT) in measurement of HaemoglobinIndian J Comm Health 2017 30(1):72-79. [Google Scholar]