Type 1 Diabetes (T1D) results from insulin deficiency due to impaired secretion of insulin from pancreas (insulin dependent diabetes mellitus) which may be autoimmune or idiopathic in origin [1]. Previous work by Brownlee M reports the association between diabetes and oxidative stress [2,3]. Reactive Oxygen Species (ROS) can cause strand breaks in DNA which result in base modifications by oxidation of guanine residues to 8-hydroxy-2’deoxyguanosine (8-OHdG) [4]. Oxidative damage to DNA can be estimated by measuring levels of 8-OHdG, a biomarker of oxidant-induced DNA damage, in mononuclear cells from diabetic subjects [5]. Various clinical studies report the role of 8-OHdG in relation to oxidative stress and diabetes mellitus [6]. Increased basal levels of DNA damage biomarkers in whole blood from the T1D patients suggests the involvement of hyperglycaemia in oxidative DNA damage [7,8]. An association between diabetes and DNA damage has also been suggested in a similar study conducted in our department on type 2 diabetes patients. These diabetic patients had lower vitamin D levels which may increase DNA damage [9]. The present study was designed to assess the level of DNA damage among patients with T1D and to ascertain the relationship between 8-OHdG levels and the oxidative stress markers: Malondialdehyde (MDA) and Total Antioxidant Capacity (TAC).
Materials and Methods
This observational case-control study was carried out at the Duhok Diabetes Center and Clinical Biochemistry Department of Azadi General Teaching Hospital, from September 2017 to March 2018. Medical Ethics committee approval (IEC Reference No.: 07062016-4) and informed written consent from parents was obtained for all the participants. A total of 255 subjects aged 8-18 years living in Duhok governorate, Kurdistan region, (Iraq) were included in the study. Among these, 132 participants were diabetic patients (T1D) with duration of disease ranging from 2 to 6.3 years attending the diabetes centre during the period of the study, and 123 apparently healthy age and sex matched controls were selected from the primary and secondary schools in Duhok governorate. Selection of cases and healthy controls was carried out using random sampling technique. The sample size for this study was calculated with 80% power at a 5% level of statistical significance.
Data Collection
A pre-tested questionnaire was designed to obtain information on gender, age (recorded to the nearest year), weight (recorded to the nearest kilogram) using a usual non-electronic scale (detectoscale) and height (recorded to the nearest centimeter) using the same scale. BMI was calculated for each subject. Diabetic patients with history of cardiovascular, respiratory, rheumatoid, renal and hepatic diseases, malignancy or recent infections were excluded from the study. Apparently healthy subjects with no history of chronic diseases or history of diabetes mellitus among first degree relatives were recruited as the control group.
Collection of Blood Samples
Participants were instructed to avoid any heavy physical activity for more than two hours before the examinations. Blood samples after overnight fasting for 12-14 hours were collected between 9:00-11:30 am at the Lab-Department of Clinical Biochemistry at Azadi General Teaching Hospital. About 10 mL of blood was withdrawn by venipuncture, using VACUTAINER from the antecubital vein and collected in BD Vacutainer System CAT- plain tubes. The sera were separated by centrifugation using a (HITACHI centrifuge, Model O5P-21) at 5000 rpm for 10 min at room temperature and collected into two tubes, one processed immediately for measuring serum MDA, TAC and 8-OHdG. Standard colorimetric analysis was done to estimate MDA and TAC were at 532 nm and 570 nm respectively [10,11]. Serum 8-OHdG was estimated using Enzyme-Linked Immunosorbent Assay (ELISA) kit (Catalog number: E-EL-0028, ELABSCIENCE. USA). The remaining sera were stored at –80°C until further analysis. Insulin Autoantibodies (IAA) were measured using Radioimmunoassay (RIA) kit (Catalog number: MG13041, IBL international GMBH, D-22335 Hamburg, Germany), Islets cell antibodies (ICA) using ICA screening ELISA kit (RSR diagnostic for autoimmunity Catalog number: c2GI/96; RSR Limited ParcTyc Glas Llanishen, Cardiff, CF145DU U.K) and Glutamic Acid Decarboxylase Antibodies (GADA) using RSR GAD65 autoantibody (GADA) ELISA kit (Avenue Park Pentwyn Cardiff CF23 8HE United Kingdom). Assessment of DNA damage was based on the levels of 8-OHdG, subjects with >4.0 ng/mL were considered to have high level of DNA damage.
Statistical Analysis
All data were analysed using the Statistical Package for Social Sciences (SPSS) version 18.0 computer software. Descriptive statistics were adapted to present data as mean±SD. Independent t-test and un-paired student’s t-test were used to assess differences in serum analyte among groups for continuous data. Significance of association between various risk factors for categorical data was assessed by using Chi-square test for association between two groups and one-way ANOVA test for association among more than two groups. Pearson’s correlation coefficient was used to assess the relationship between serum 8-OHdG level and TAC. Level of statistical significance was set at p<0.05.
Results
The primary objective of the study was to find out the levels of 8-OHdG in T1D patients in comparison with healthy subjects. Out of all the132 diabetic cases included in this study, 87 (65.9%) patients were found to have high level of DNA damage as compared to 12 (9.8%) healthy controls.
An 8-OHdG levels were found to be significantly increased in patient group as compared to healthy controls, (p<0.01). For this purpose, MDA was estimated in both groups and was found to be significantly increased in diabetic patients as compared to healthy controls (p=0.01). TAC was also estimated and was found to be significantly decreased in diabetic patients as compared to healthy controls, (p=0.01) [Table/Fig-1].
Demographic and clinical characteristics of all participants under study.
| Variable | Patients (n=132) | Controls (n=123) | p-value |
|---|
| Age (years)* | 14.6±2.2 | 16.2±3.1 | 0.68 |
| Female sex, n (%) | 81 (61.4) | 75 (61.0) | 0.95 |
| Body Mass Index (Kg/m2)* | 20.2±3.6 | 23.5±2.7 | 0.25 |
| Duration of disease (years) | 4.2±2.1 | -- | -- |
| Autoantibodies, n (%) | 87 (65.9) | -- | -- |
| 8-hydroxy-2’deoxyguanosine (ng/mL)* | 6.02±2.05 | 2.03±1.63 | <0.01** |
| Malondialdehyde (nmol/L)* | 1.52±0.65 | 1.25±0.09 | 0.01** |
| Total Antioxidant Capacity (mmol/L)* | 1.29±0.16 | 1.91±1.03 | 0.01** |
| DNA damage, n (%) | 87 (65.9) | 12 (9.8) | <0.01** |
*Results are mean±SD, **Chi-square test and Independent t-test used with p<0.05 set as significant cut-off value
To evaluate the relationship of autoantibodies and 8-OHdG levels, all patients were divided into 2 groups (with and without autoantibodies). An 8-OHdG levels in the patient group with autoantibodies were higher than that in the patient group without autoantibodies, with (p=0.04). Significantly, lower BMI (p=0.02) and higher duration of disease (<0.01) values were observed in the patient group with autoantibodies. However, no significant difference was observed between groups with respect to oxidative stress biomarkers (MDA and TAC) [Table/Fig-2].
Serum 8-hydroxy-2’deoxyguanosine sratified by Autoantibodies.
| Variable | Patients without Autoantibodies (n=45) | Patients with Autoantibodies (n=87) | p-value |
|---|
| Age (years)* | 12.5±2.5 | 15.7±3.6 | 0.08 |
| Female sex, n (%) | 30 (66.6) | 51 (58.6) | 0.17 |
| Body Mass Index (Kg/m2)* | 22.1±3.6 | 19.3±3.5 | 0.02** |
| Duration of disease (years)* | 3.4±1.7 | 4.7±2.0 | <0.01** |
| 8-hydroxy-2’deoxyguanosine (ng/mL)* | 5.7±1.9 | 6.2±2.1 | 0.04 |
| Malondialdehyde (nmol/L) | 1.51±0.62 | 1.53±0.69 | 0.90 |
| Total Antioxidant Capacity (mmol/L)* | 1.25±0.11 | 1.32±0.22 | 0.25 |
*Results are mean+SD, **Chi-square test and Independent t-test used with p<0.05 set as significant cut-off value
Data were further analysed to determine the relationship between autoantibodies and DNA damage. In patient group with autoantibodies, DNA damage above cut-off value of serum 8-OHdG of 4.0 ng/mL was 79.3%, whereas in the patient group without autoantibodies was 40.0%, with p=0.02 [Table/Fig-3].
Distribution of type 1 diabetes patients according to DNA damage.
| Autoantibodies | n | 8-hydroxy-2’deoxyguanosine | p-value |
|---|
| <4.0 | >4.0 |
|---|
| n (%) | n (%) |
|---|
| Insulin autoantibodies | 51 | 9 (17.7) | 42 (82.3) | 0.41 |
| Islet cells antibodies | 24 | 6 (25.0) | 18 (75.0) |
| Glutamate decarboxylase autoantibodies | 12 | 3 (25.0) | 9 (75.0) |
| With Autoantibodies (all) | 87 | 18 (20.7) | 69 (79.3) | 0.02* |
| Without Autoantibodies | 45 | 27 (60.0) | 18 (40.0) |
* Chi-square test used with p<0.05 set as significant cut-off value
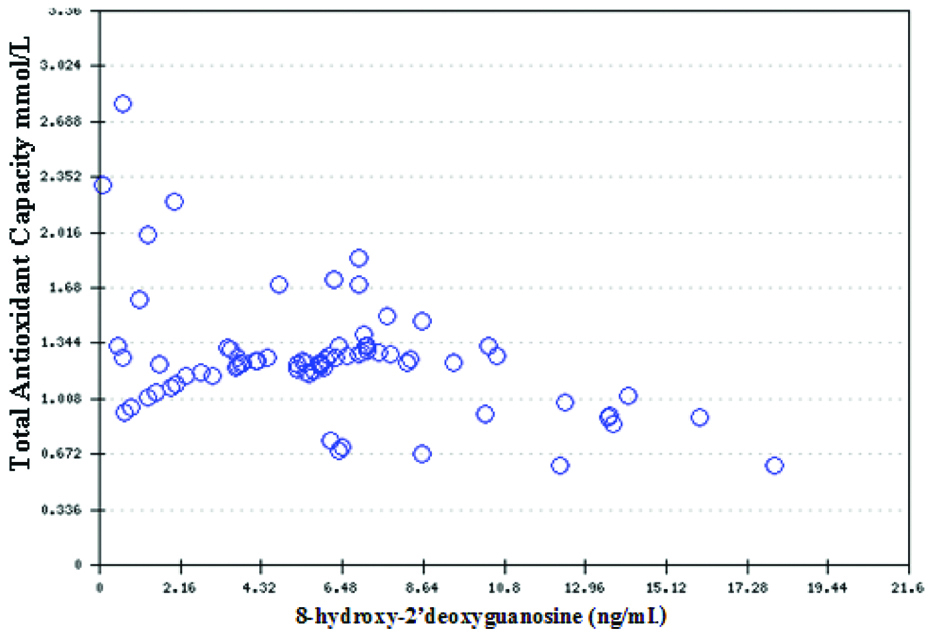
In the patient group, 8-OHdG negatively correlated with TAC (r=-0.43, p=<0.01) and positively with MDA (r=0.17, p=0.09) which indicates that oxidative DNA damage increases as the oxidative stress biomarker level rises [Table/Fig-4].
A Pearson’s correlation of serum 8-hydroxy-2’deoxyguanosine level with TAC, r=-0.43, p<0.01.
Discussion
In this study, 8-hydroxy 2-deoxyguanosine (8-OHdG) has been used as a marker for assessment of DNA damage. Other parameters that have been used include MDA and TAC. While MDA (which is a marker of oxidative damage) is used for assessing the degree of lipid peroxidation of the cell membranes in diabetes; TAC is used to estimate the degree of counteraction of the oxidative damage by the antioxidants. These two parameters have been used to find their relationship with DNA damage. Moreover, the role of autoantibodies on DNA damage has also been assessed. The findings are very important and shed new light on the role of DNA damage in type 1 diabetic patients.
In the present study, we found that patients with T1D have higher level of DNA damage represented by high serum 8-OHdG levels than healthy individuals. Our results in combination with previously published results, suggest that serum 8-OHdG level is a potentially useful biomarker for evaluating the severity of DNA damage, particularly in patients with diabetes mellitus [12,13]. Results of the present study also showed that the DNA damage is highly prevalent in diabetic patients (65.9%) as compared to healthy controls, although 9.8% of the healthy subjects also showed a high degree of DNA damage. To our knowledge, the present study is the first to examine the individual and collective effects of oxidative stress and other related factors on the level of DNA damage in T1D patients and healthy individuals in Duhok, Kurdistan region (Iraq).
Several studies demonstrate that the formation of free radicals (ROS) through oxidative stress process is mediated mainly by hyperglycaemia. These free radicals are thought to be mediated in the process of oxidative DNA damage. On the contrary, there are controversies that regarding the role of these free radicals as a reliable marker of oxidative stress or markers of DNA damage in clinical practice [14,15]. Our study has provided definitive evidence that T1D have a high level of DNA damage. The best relationship of high serum 8-OHdG levels was with low TAC. It is noteworthy that 99 (75.7%) of studied subjects appear to be at risk of DNA damage and most of them 87 (87.9%) were diabetic patients. Though, other factors may play an important role in initiation of oxidative stress and progression of the DNA damage, these processes are thought to be counter acted by short and/or long-term effects of antioxidants [16].
T1D is primarily due to deficiency of insulin that is sub classified as autoimmune T1Da or idiopathic (T1Db) [1]. Presence of diabetes antibodies such as GADA, ICA and IAA, mostly suggests that T1D is an autoimmune process [12]. It has generally been considered that autoantibodies function as only markers of disease and have no role in the pathogenesis. The measurement of islet autoantibodies helps in diagnosis of autoimmune diabetes. Presence of these autoantibodies in non-diabetic individuals indicates increased risk T1D development [17]. In the present study, we measured autoantibodies in serum of selected T1D patients and showed that the most frequent autoantibodies was IAA followed by ICA and GADA. The patients with autoantibodies had higher mean 8-OHdG levels and longer duration of the disease as compared to patients without antibodies. This, association between DNA damage and duration of the disease indicates that oxidative damage is linked to the metabolic changes that occur in prolonged duration of the disease instead of the presence of autoantibodies.
Limitation
Further studies on different population with larger sample size and more than one marker of oxidative stress as well as for DNA damage are required to confirm association between oxidant-antioxidant balance and DNA damage.
Conclusion
T1D patients have more severe oxidative stress condition and oxidative DNA damage than healthy subjects, suggesting that increased oxidative stress may be associated with the progression of diabetes overtime. Preventive intervention in order to reduce the tendency of DNA damage among diabetic patients should focus on the awareness of the antioxidant supplementation.
*Results are mean±SD, **Chi-square test and Independent t-test used with p<0.05 set as significant cut-off value
*Results are mean+SD, **Chi-square test and Independent t-test used with p<0.05 set as significant cut-off value
* Chi-square test used with p<0.05 set as significant cut-off value