Introduction
Angiogenesis is a major part of a variety of physiological and pathological processes, including tumour growth. Vascular Endothelial Growth Factor (VEGF) is a highly potent and specific angiogenic factor. Many studies have indicated the importance of VEGF in growth and expansion of lesions which contributes to the local invasion of odontogenic lesions.
Aim
To investigate the expression of VEGF in Ameloblastoma and Dentigerous Cyst, using a monoclonal antibody, then compare between them to investigate the role in diagnosis and determination of biological behaviour of some odontogenic lesions.
Materials and Methods
The sample consisted of 15 formalin-fixed, paraffin-embedded specimens of Ameloblastoma (Follicular-plexiform pattern) and 15 cases of Dentigerous Cyst that were conventionally stained with H and E and immunohistochemically stained with a monoclonal anti-VEGF antibody. VEGF expression in this specimen was qualitatively evaluated, calculating the percentage of VEGF positive cells in the epithelial and stromal cells.
Results
The expression of VEGF in Ameloblastoma (Follicular-plexiform subtype) was varied between high and medial while its expression in Dentigerous Cyst was low, the statistical analysis indicated significant differences between these lesions (p<0.05).
Conclusion
VEGF contributes to the invasion of aggressive odontogenic lesions, thus we concluded its role as diagnostic and prognostic markers in the prediction of the biological behaviour of odontogenic lesions.
Introduction
Odontogenic lesions constitute an important aspect of oral and maxillofacial pathology [1]. Ameloblastoma (AML) is a locally aggressive benign epithelial odontogenic tumour with a marked invasion potential that results in multiple recurrences after treatment [2]. Ameloblastoma occurs at any age but often in the third-fourth decade of life. According to WHO classification (2017) ameloblastoma was divided into solid or multicystic type, unicystic type, peripheral type [3].
The clinical signs of the ameloblastoma are changeable, but they are commonly associated with painless mandibular expansion because of their slow growth. Pain and numbness are rare symptoms [4].
Histologically, AML is formed by odontogenic epithelium with fibrous mature stroma [5] and shows different histological subtypes: follicular, plexiform, acanthomatous, desmoplastic, granular cell, and basal cell subtypes but the first two being the most common [6].
Follicular type has a high recurrence rate and is composed of many islands which contain peripheral columnar cells (ameloblast like cells) and central cells (stellate reticulum like cells), whereas the epithelial tumour cells in plexiform type are arranged in double rows of columnar cells (like a network).
Dentigerous cyst (DC, follicular cyst) is known as the most common odontogenic developmental cyst that originates by the separation of the follicle from around the crown of an impacted tooth originating from dental follicle [7]. In addition, DC is classified as the second most common odontogenic cyst after periapical cysts [8].
Most of the cysts are detected early and the surgical treatment can adequately solve the clinical problems but sometimes the cyst can grow significantly large and their resection causes the need of reconstruction and can lead to clinical challenges. Also, tumours transformation occurring in DC have been reported but is rare [9], few cases found in medical literature showed the neoplastic transformation of the epithelium of dentigerous cysts to unicystic ameloblastoma, epidermoid carcinoma, and mucoepidermoid carcinoma [10].
Pinheiro JJ et al., in their study on ameloblastoma aggressiveness explored that almost half of ameloblastoma cells make protein [11]. Such protein activity may participate in a mechanism that contribute in the growth pattern of ameloblastoma. This activity could include proteolytic proteins related to the invasive phenotype.
Angiogenesis is a major part of a variety of physiological and pathological processes, including, inflammation and tumour growth, and is known as the process by which new blood vessels are produced by increasing from pre-existing vasculature. This process depends on strictly controlled endothelial cell proliferation, migration and survival, and is regulated by various proteins, most of which are polypeptide growth factors [12]. The angiogenic factors play a role as essential mediators of tumour angiogenesis as in epithelial odontogenic tumours and the changes might be associated with tumourigenesis or malignant diversion of odontogenic lesions. The proangiogenic cytokine is capable of stimulation of microvascular permeability leading to extravasation of plasma proteins, and an expected sequence of proangiogenic stromal changes. Due to these functions, VEGF has been implicated as an essential factor in granulation tissue development and tumour growth [13].
VEGF’s is a family of multifunctional proteins mainly involved in normal and pathologic angiogenesis, defined as the formation of new vessels by sprouting of the pre-existing endothelium. The VEGF family includes VEGFA (was known as vascular permeability factor VPF), VEGFB, VEGFC, VEGFD, VEGFE, and placental growth factors. VEGF is a cytokine and a dimeric heparin-binding glycoprotein with an eclectic mitogenic effect on vascular endothelial cells, VEGF is a highly potent and specific angiogenic factor [13].
Many studies in odontogenic lesions have been done to assess the expression of VEGF. But a few of them have been evaluated as epithelial and mesenchymal components. Therefore, the aim of the present study is to assess VEGF expression in ameloblastoma and dentigerous cyst subsequently to understand more about the aggressive behaviour of ameloblastoma.
Materials and Methods
A retrospective study was carried out comprising a total of 30 cases, included 15 formalin-fixed, paraffin-embedded blocks of ameloblastoma (10 follicular type, 5 plexiform type), 15 other of dentigerous cyst without any inflammation obtained from archives of the Department of Oral and Maxillofacial Pathology in The Damascus University, between January 2015 and July 2016. Ethical Committee approval was obtained.
In the present study G POWER was used for sample size calculation and we got 88% power of 30 cases.
Exclusion Criteria
Exclusion criteria were patients having any immunological and systemic diseases, any other oral lesions along with cysts and tumours, tobacco habits, and any inflammatory dentigerous cysts.
A 3 cm3 paraffin section of formalin-fixed tissues were used for both routine (H and E) stained sections and Immunohistochemical (IHC) examination. Histological (H and E) stained sections were used for the initial diagnosis. For immunohistochemical evaluation, 4 μ sections were made and loaded on slides. Slides were deparaffinised and washed in deionised water and treated with antigen retrieval. Peroxide block (3% hydrogen peroxide) was used to block endogenous peroxidase for 12 minutes.
After pre-treatment, sections were incubated with primary antibody rabbit monoclonal to VEGF antibody (Bio SB, USA), in a humid chamber at 4°C overnight, performing heat mediated antigen retrieval with citrate buffer at pH 6 before commencing with IHC staining protocol.
The standard streptavidin-biotin-peroxidase complex method was performed to bind the primary antibodies (biogenex Life Sciences Ltd., CA, USA). The reaction ingredients were visualised by treating with diaminobenzidine solution diluted according to the manufacturer’s instructions.
Staining Evaluation
For positive control, sections of the placenta for VEGF were taken and treated in a similar manner as the test sample with each batch of staining, and they were run with each batch of staining.
The IHC expression of VEGF was studied in both the epithelial cells as well as connective tissue of AML and DC (fibroblast, endothelial cells of micro vessels, inflammatory cells). As VEGF is a cytoplasmic markers, every epithelial and stromal cell having diffuse brown staining in cytoplasm of the cell, was considered positive.
Light microscopy under 100X magnification was used and four hot spots with the largest number of immunostained cells were identified. Further, 400X magnification was used for counting the number of immunopositive cells in these fields.
The IHC staining was evaluated using qualitative method based on an adaptation of the criteria of Leonardi R et al., which divided the result to four groups: group 0- no staining; group 1- weak staining in 11-25% of cells; group 2- moderate staining in 26-75% of cells; group 3- strong staining in more than 76% of cells [14].
Statistical Analysis
The results were computed and subjected to statistical analysis using T-student (for independent samples) test. Statistical analysis indicated significant differences between AML and DC (p-value<0.05).
Results
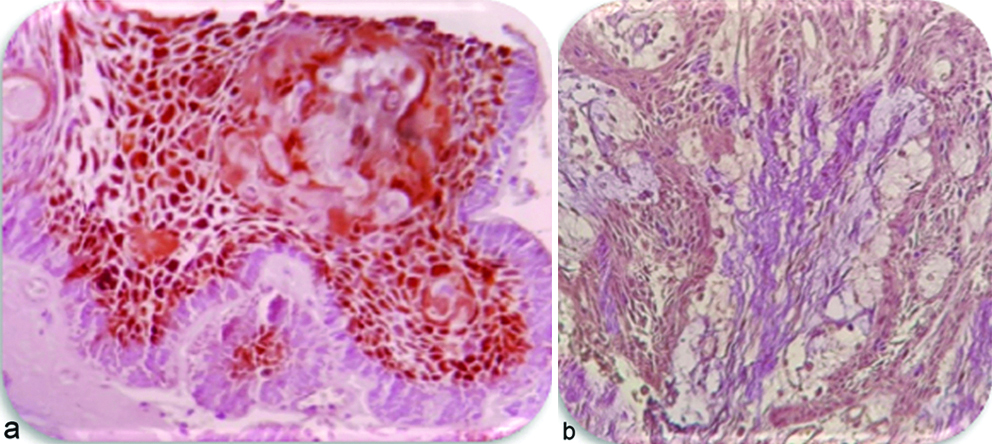
Vascular Endothelial Growth Factor (VEGF): Five cases of AML were strongly positive in epithelial cells, especially stellate reticulum like cells, seven cases expressed moderate positive in ameloblast like cells and stellate reticulum like cells, three cases expressed weak positive in neoplastic odontogenic epithelial cells [Table/Fig-1a,b]. VEGF was also present in stromal cells but the expression in the stromal cells (fibroblast, endothelial cells, inflammatory cells) was not as strong as in the epithelial cells [Table/Fig-2].
Expression of VEGF (400). Note the diffuse staining in ameloblastoma in (a) follicular type: high percentage was observed in the central cells of follicle, group-3 (b) plexiform type, group-3.
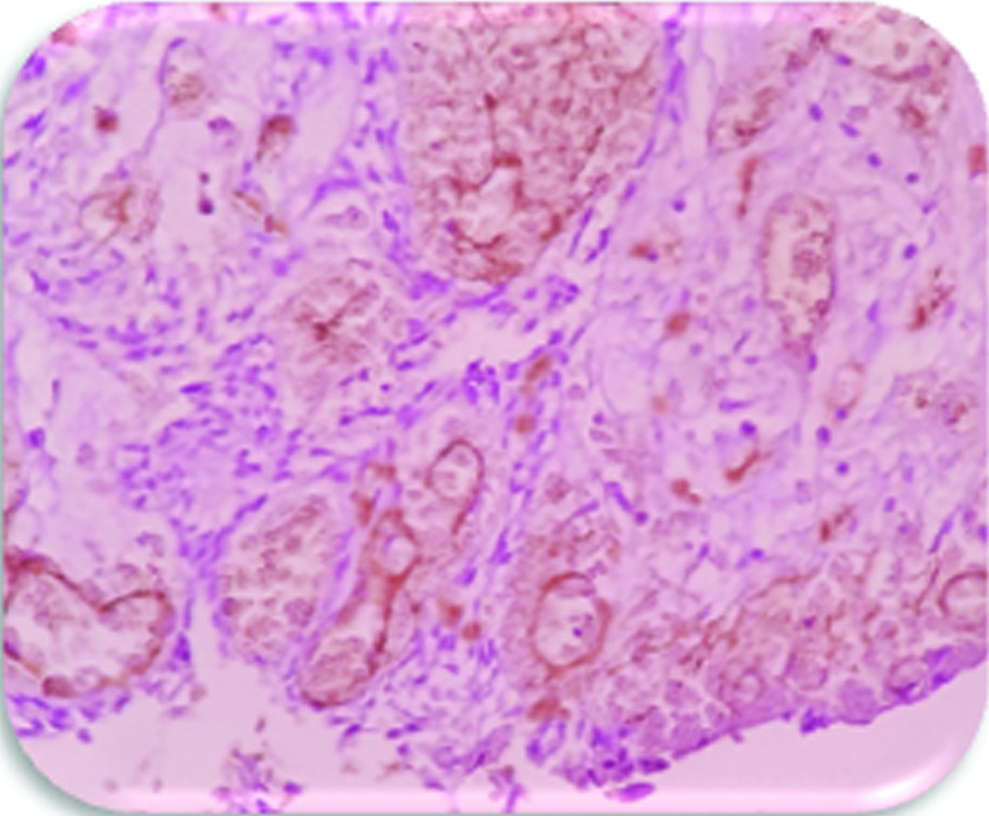
Expression of VEGF in stroma of AML (400X). Note expression in stromal cells especially endothelial cells of microvessels, group-2.
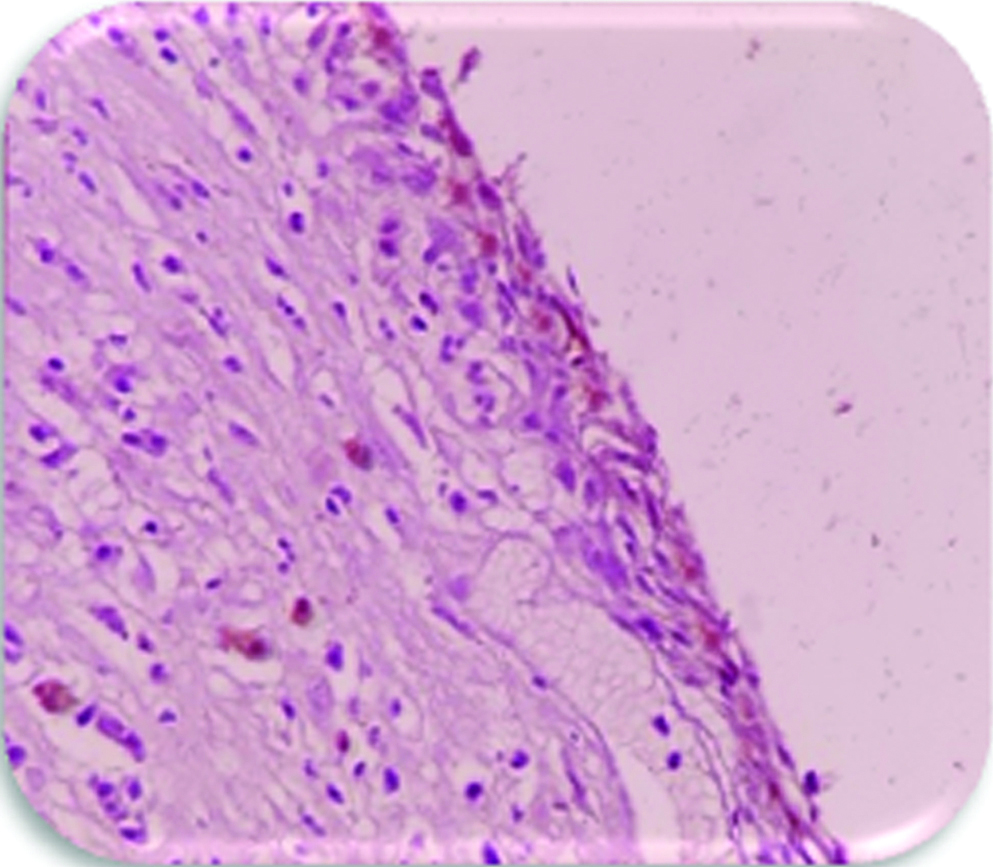
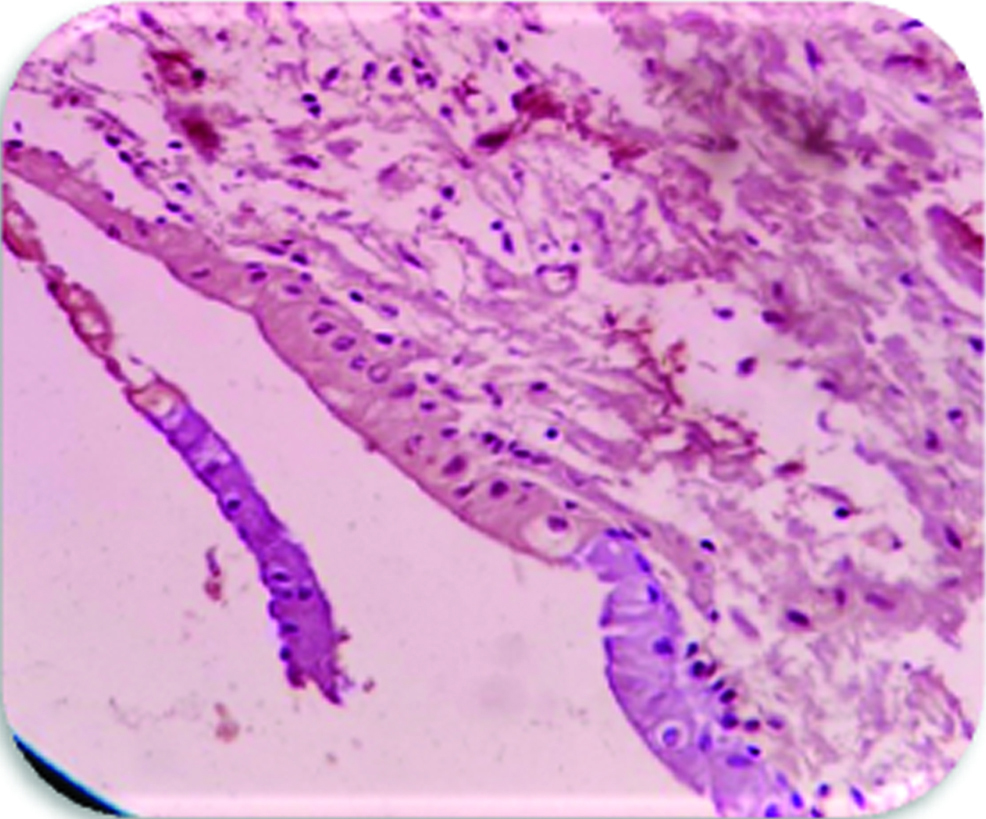
Most cases of DC (10/15) expressed weak staining in epithelium and connective tissue [Table/Fig-3]. Four cases showed moderate staining [Table/Fig-4] and only one case was showing strong staining in epithelium and connective tissue.
Expression of VEGF in the epithelium and connective tissue of dentigerous cyst (weak staining) (DC) (400X), group-1.
Expression of VEGF in the epithelium and connective tissue of dentigerous cyst (moderate staining) (400X), group-2.
Discussion
The diagnosis and assessment of prognosis of pathological lesions are very important tools to every pathologist, hence the recent studies emphasise that tumour markers are supposed to play a progressively main role as adjuvant tools [15].
Gupta B et al., have suggested that VEGF expression may be an important marker in the diagnosis of angiogenesis in developing pre-cancerous and invasive lesions [16].
Ameloblastoma (AML) is known as an odontogenic epithelial tumour which is benign and locally aggressive with destructive morbidity if left without treating, due to its limitless growth potential. Since the treatment is radical surgical intervention and long-term follow-up, diagnosis and assessment of prognosis is essential [4].
A lot of factors have been associated with the aggressive behaviour and invasive nature of odontogenic lesions, such as an increased proliferation potential, changes in the expression of tumour suppressor genes, and the abnormal expression of cell cycle-regulating proteins, adhesion molecules [17].
VEGF expression in odontogenic neoplastic epithelial cells of ameloblastoma has been described by Kumamoto H et al., but the localisation was different from the present study as they showed higher VEGF expression by the peripheral cells of ameloblastoma (ameloblast-like cells) as compared to central cells (stellate reticulum like cells) [12]. But according to Kumar H et al., cyclin D1 showed expression in both central and peripheral cells in both follicular and plexiform ameloblastoma [18], Yang SX et al., showed that there is a positive correlation between VEGF and cyclin D1 in a lot of tumours [18,19] which shows same staining pattern as seen in the present study and VEGF expression is seen in many different types of carcinomas. Consequently, in the present study, the higher VEGF expression in the central cells of the follicles as compared to ameloblast-like cells may due to different reasons which are discussed above irrespective of the type of the antibody used.
Dentigerous Cyst has a good prognosis and low recurrence rate and forms 20% of oral cysts [20], The low expression in our cases reflected the fact that VEGF is associated with the biological behaviour of odontogenic lesions. This was in accordance with previous studies [9,20].
When the data of positive cells for VEGF in the epithelial cells of studied lesions was compared, it was found that the expression of AMLs as compared with DCs, showed extremely significant results p<0.05, [Table/Fig-5].
Difference between the mean values of VEGF in AML and DC.
| Type of cells | T-value | Differences between mean values | p-value | |
|---|
| Expression of VEGF | Epithelial cells | 3.144 | 26.27 | 0.004 | Significant differences |
| Stromal cells | 4.109 | 25.20 | 0.000 | Significant differences |
Gupta B et al., also had same results as in the present study that showed higher expression positivity of VEGF in the epithelial lining of ameloblastoma as compared to dentigerous cysts [21].
Data of the present study reveals that most of cases showed VEGF expression extremely or moderately in the stroma (fibroblast, endothelial cells, inflammatory cells) of AMLs. This was significantly more as compared to DCs which showed low expression p<0.05, [Table/Fig-5].
Studies investigating the expression, of VEGF in odontogenic lesions are scarce. In view of ameloblastomas aggressive biologic behaviour, studies have tried to identify the molecular basis underlying the distinct behaviour of AML’s compared with other odontogenic lesions [17]. The present study has evaluated the VEGF in the epithelium, stroma of AML and DC.
In subtypes of ameloblastomas, Kumamoto H et al., found no significant difference in VEGF expression between follicular and plexiform ameloblastomas but in the present study, VEGF expression was significantly increased in the stroma of plexiform type p<0.05, [Table/Fig-6] [12]. This contradiction may due to the difference in number of samples which may affect statistically.
Difference between the mean values of VEGF in follicular and plexiform ameloblastoma.
| Type of cells | T-value | Differences between mean values | p-value | |
|---|
| Expression of VEGF | Epithelial cells | -0.545 | -7.80 | 0.595 | Significant differences |
| Stromal cells | -2.309 | -22.80 | 0.038 | Significant differences |
Limitation
In the present study, more required cases in the archives could not be found, due to the scarcity of biopsies for these lesions.
Conclusion
The results of this study can support the hypothesis that angiogenesis could be an important mechanism in the aggressiveness and the growth of ameloblastoma. The role of vascular endothelial growth factor in the pathogenesis of odontogenic lesions should be further evaluated as angiogenesis could be a potential goal for the therapeutic management of these lesions. This protein can be used as a diagnostic and prognostic marker for biological behaviour of odontogenic tumours. The expression of VEGF in odontogenic lesions should be further evaluated with a larger sample size for a better understanding of the role of these markers in pathogenesis of lesions.
[1]. Neville B, Douglas D, Carl M, Bouquot J, Odontogenic cysts and tumoursOral and Maxillofacial Pathology 2009 3rd Edition:589-637. [Google Scholar]
[2]. Wang A, Zhang B, Huang H, Zhang L, Zeng D, Tao Q, Suppression of local invasion of ameloblastoma by inhibition of matrix metalloproteinase-2 in vitroBMC Cancer 2008 8:18210.1186/1471-2407-8-18218588710 [Google Scholar] [CrossRef] [PubMed]
[3]. Soluk-Tekkesin M, Wright JM, The World Health Organization Classification of Odontogenic Lesions: A Summary of the Changes of the 2017 (4th) EditionTurk patoloji dergisi 2018 34(1) [Google Scholar]
[4]. Zakaraia S, Almohareb M, Zaid K, Doumani M, Seirawan MY, Amelogenin is a potential biomarker for the aggressiveness in odontogenic tumoursAsian Pac J Cancer Prev 2018 19(5):1375-79. [Google Scholar]
[5]. Anne R, Krisnuhoni E, Chotimah C, Latief BS, Matrix metalloproteinase-9 (mmp-9) expression in different subtypes of ameloblastomaJ Maxillofac Oral Surg 2014 13(3):281-85.10.1007/s12663-013-0538-z25018601 [Google Scholar] [CrossRef] [PubMed]
[6]. Zhong Y, Guo W, Wang L, Chen X, Molecular markers of tumour invasiveness in ameloblastoma: An updateAnn Maxillofac Surg 2011 1(2):145-49.10.4103/2231-0746.9278023482687 [Google Scholar] [CrossRef] [PubMed]
[7]. Khot K, Deshmukh SB, Alex S, Comparative analysis of the immunohistochemical expression of vascular endothelial growth factor and matrix metalloproteinase-9 in keratocystic odontogenic tumour, dentigerous cyst and radicular cystJ Cancer Res Ther 2015 11(3):635-40.10.4103/0973-1482.14459126458594 [Google Scholar] [CrossRef] [PubMed]
[8]. Friedlander LT, Hussani H, Cullinan MP, Seymour GJ, De Silva RK, De Silva H, VEGF and VEGFR2 in dentigerous cysts associated with impacted third molarsPathology 2015 47(5):446-51.10.1097/PAT.000000000000027226126033 [Google Scholar] [CrossRef] [PubMed]
[9]. Suojanen J, Lehtonen N, Farkkila E, Hietanen J, Teronen O, Sorsa T, Common Matrix Metalloproteinases (MMP-8, -9, -25, and -26) cannot explain dentigerous cyst expansionJ Clin Diagn Res 2014 8(9):ZC82-85.10.7860/JCDR/2014/9221.489925386530 [Google Scholar] [CrossRef] [PubMed]
[10]. Kondamari SK, Taneeru S, Guttikonda VR, Masabattula GK, Ameloblastoma arising in the wall of dentigerous cyst: Report of a rare entityJ Oral Maxillofac Pathol 2018 22(Suppl 1):S7-S10.10.4103/jomfp.JOMFP_197_1529491596 [Google Scholar] [CrossRef] [PubMed]
[11]. Pinheiro JJ, Freitas VM, Moretti AI, Jorge AG, Jaeger RG, Local invasiveness of ameloblastoma. Role played by matrix metalloproteinases and proliferative activityHistopathology 2004 45(1):65-72.10.1111/j.1365-2559.2004.01902.x15228445 [Google Scholar] [CrossRef] [PubMed]
[12]. Kumamoto H, Ohki K, Ooya K, Association between vascular endothelial growth factor (VEGF) expression and tumour angiogenesis in ameloblastomasJ Oral Pathol Med 2002 31(1):28-34.10.1046/j.0904-2512.2001.10061.x11896820 [Google Scholar] [CrossRef] [PubMed]
[13]. Khajuria N, Metgud R, Naik S, Lerra S, Tiwari P, Mamta , Immunohistochemical expression of vascular endothelial growth factor in keratocystic odontogenic tumour, dentigerous cyst, and radicular cyst: A comparative studyIndian J Dent 2016 7(1):17-22.10.4103/0975-962X.17937827134450 [Google Scholar] [CrossRef] [PubMed]
[14]. Leonardi R, Caltabiano M, Pagano M, Pezzuto V, Loreto C, Palestro G, Detection of vascular endothelial growth factor/vascular permeability factor in periapical lesionsJ Endod 2003 29[3]:180-3.10.1097/00004770-200303000-0000412669876 [Google Scholar] [CrossRef] [PubMed]
[15]. Premalatha BR, Patil S, Rao RS, Reddy NP, Indu M, J Int Oral Health 2013 5(2):59-69. [Google Scholar]
[16]. Gupta B, Chandra S, Raj V, Gupta V, Immunohistochemical expression of vascular endothelial growth factor in orofacial lesions- A reviewJ Oral Biol Craniofac Res 2016 6(3):231-36.10.1016/j.jobcr.2016.01.00627761389 [Google Scholar] [CrossRef] [PubMed]
[17]. Henriques AC, Vasconcelos MG, Galvao HC, de Souza LB, de Almeida Freitas R, Comparative analysis of the immunohistochemical expression of collagen IV, MMP-9, and TIMP-2 in odontogenic cysts and tumoursOral Surg Oral Med Oral Pathol Oral Radiol Endod 2011 112(4):468-75.10.1016/j.tripleo.2011.05.03321911204 [Google Scholar] [CrossRef] [PubMed]
[18]. Kumar H, Vandana R, Kumar G, Immunohistochemical expression of cyclin D1 in ameloblastomas and adenomatoid odontogenic tumoursJ Oral Maxillofac Pathol 2011 15(3):283-87.10.4103/0973-029X.8668522144830 [Google Scholar] [CrossRef] [PubMed]
[19]. Yang SX, Hewitt SM, Steinberg SM, Liewehr DJ, Swain SM, Expression levels of eif4e, VEGF, and cyclin D1, and correlation of eif4e with VEGF and cyclin D1 in multi-tumour tissue microarrayOncol Rep 2007 17(2):281-87.10.3892/or.17.2.281 [Google Scholar] [CrossRef]
[20]. Sadri D, Farhadi S, Shahabi Z, Sarshar S, Expression of Vascular Endothelial Growth Factor in odontogenic cysts: is there any impression on clinical outcome?Open Dent J 2016 10:752-59.10.2174/187421060161001075228217191 [Google Scholar] [CrossRef] [PubMed]
[21]. Gupta B, Chandra S, Singh A, Sah K, Raj V, Gupta V, The role of vascular endothelial growth factor in proliferation of odontogenic cysts and tumours: An immunohistochemical studyDent Res J (Isfahan) 2016 13(3):256-63.10.4103/1735-3327.182187 [Google Scholar] [CrossRef]