MR Imaging Features of Non Convulsive Status Epilepticus: A Great Masquerader
Ayaz Jamil Ikbalhusen Dabivala1, Chetan Muljibhai Mehta2, Payal Manilal Damor3
1 Resident Doctor, Department of Radiodiagnosis, Medical College and SSG Hospital, Vadodara, Gujarat, India.
2 Professor and Head, Department of Radiodiagnosis, Medical College and SSG HospitalVadodara, Gujarat, India.
3 Assistant Professor, Department of Radiodiagnosis, Medical College and SSG Hospital, Vadodara, Gujarat, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ayaz Jamil Ikbalhusen Dabivala, 203, Lakshya Complex, Besides Hotel Subaelite, Fatehgunj Main Road, Vadodara, Gujarat, India.
E-mail: drayazdabivala1993@gmail.com
Variable clinical presentation and overlapping imaging findings make Non Convulsive Status Epilepticus (NCSE) a diagnostic challenge. NCSE is known to be a heterogeneous disorder with varied causes and several subtypes. Several disagreements exist as regards to its definition as well as the characteristic Electroencephalographic (EEG) features that are consistent with NCSE. We here present a case of nine-year-old female patient who presented with a recent onset history of regression of milestones since 5 to 6 months, and altered sensorium since 1-2 days. Contrast enhanced MRI brain was performed in this patient. This case highlights NCSE as a masquerader of inborn error of metabolism and attempts to address MR imaging findings of NCSE in acute intensive care setting.
Diffusion weighted imaging, Magnetic resonance, Wilson’s disease
Case Report
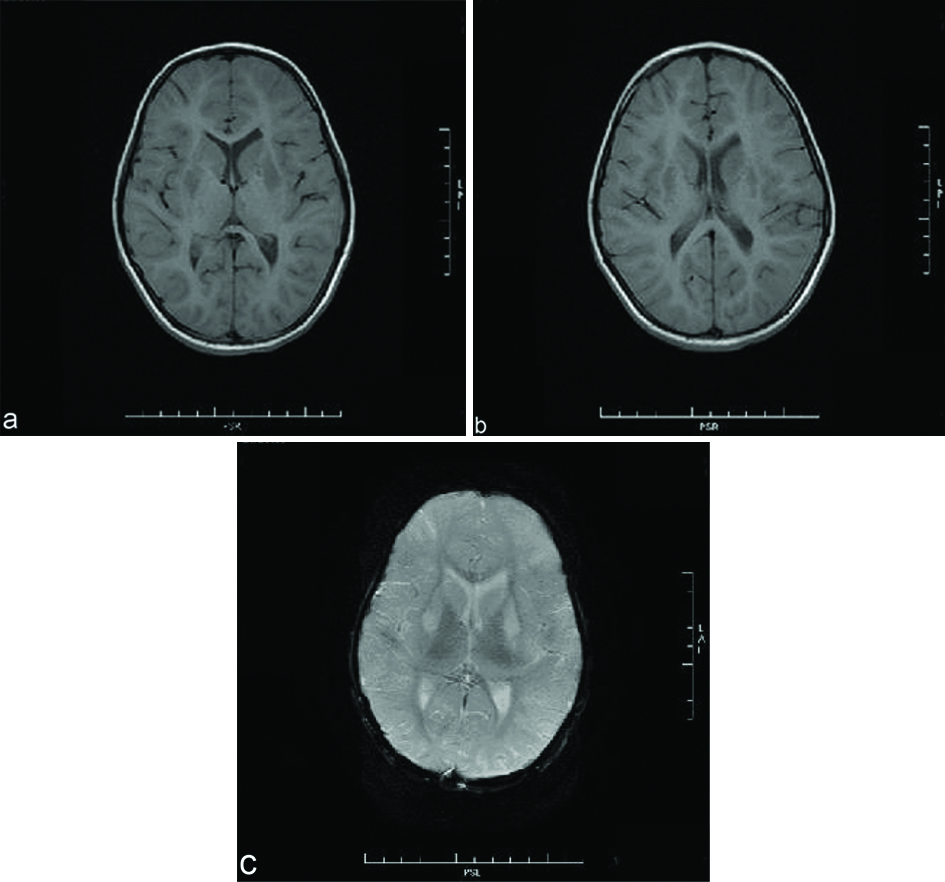
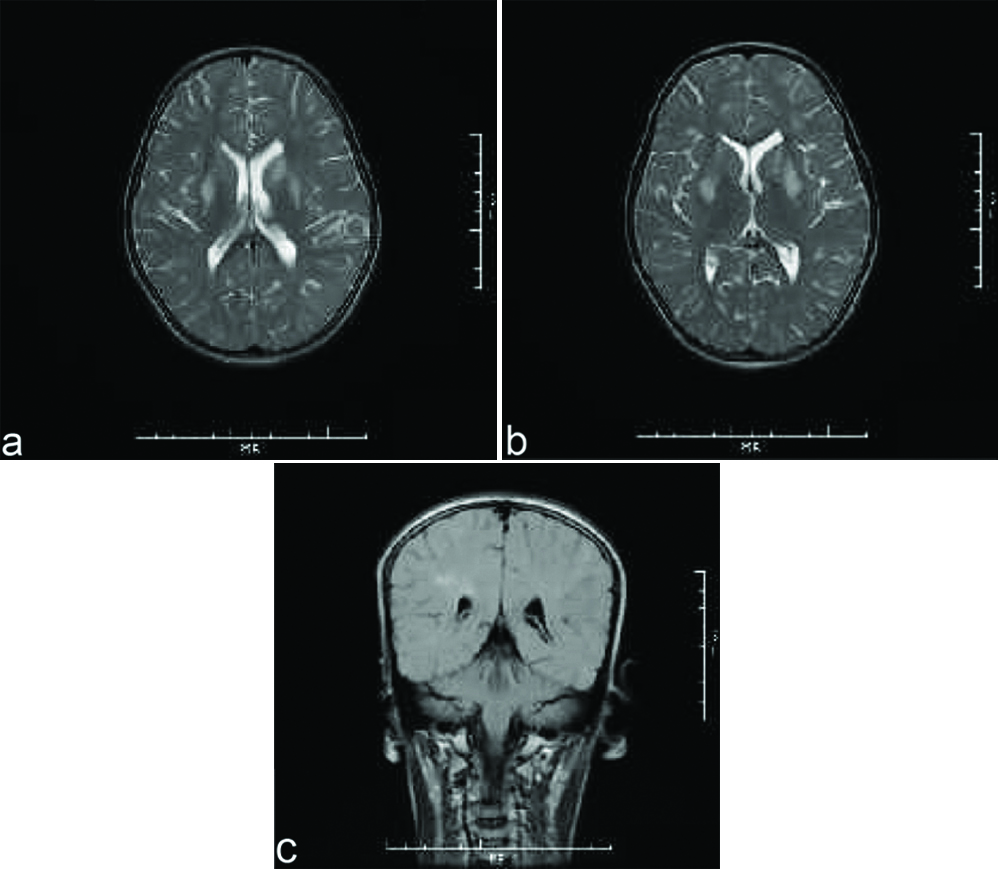
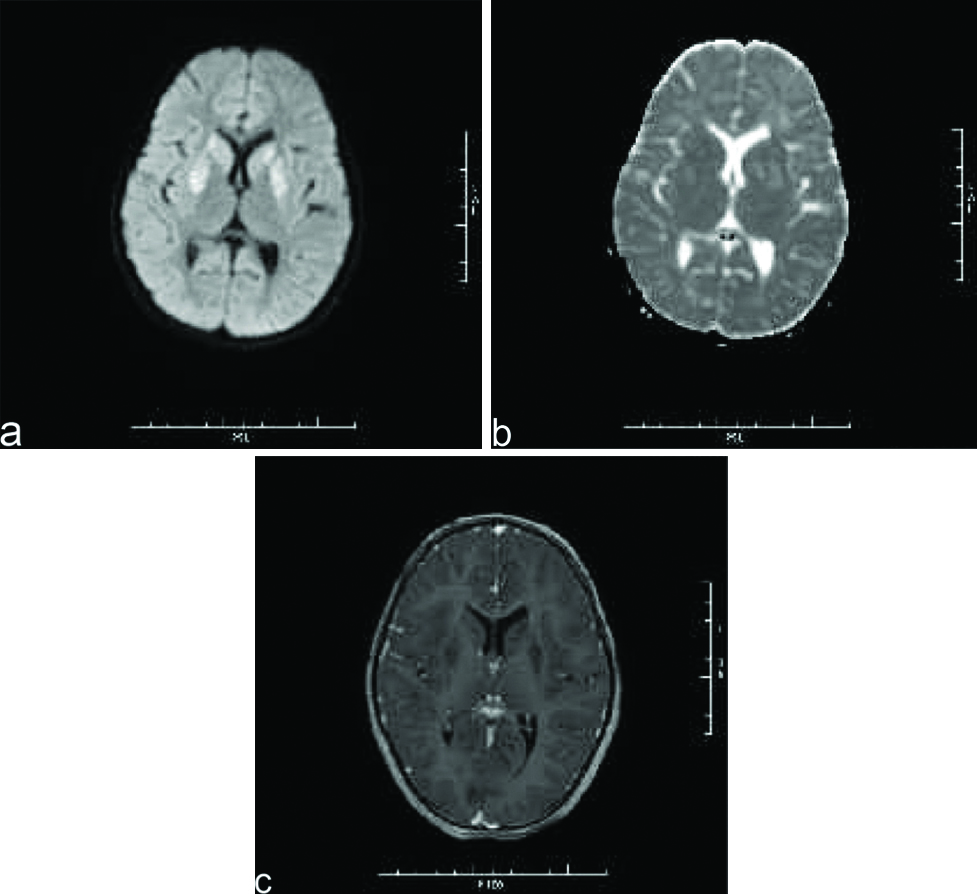
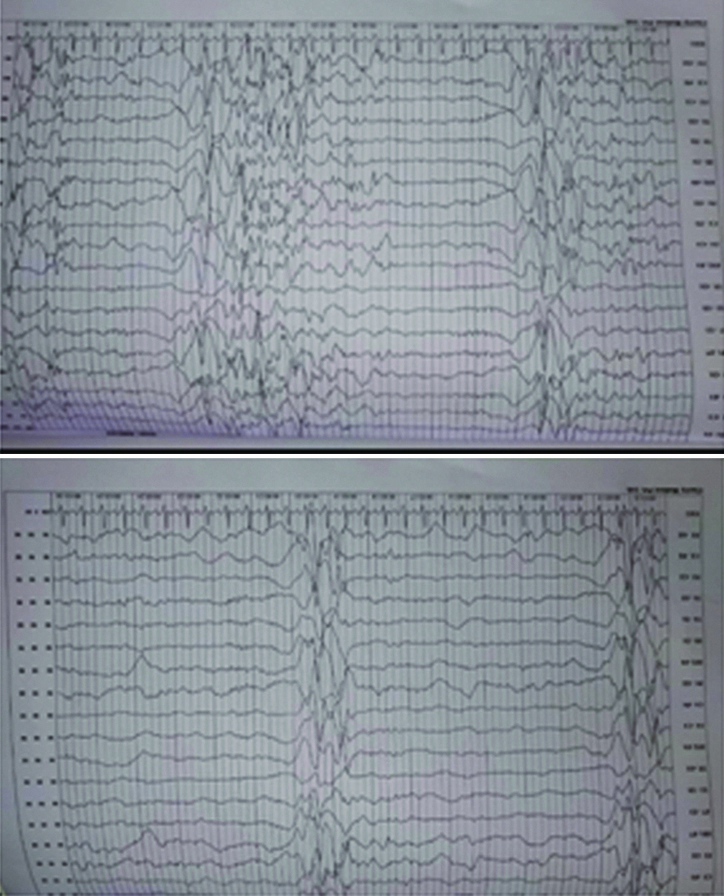
A nine-year-old female was brought to paediatric emergency with complaint of altered sensorium since 1-2 days. The child’s mother revealed on further elaboration that she had complaints of imbalance while walking since 5-6 months, and complaints of low grade fever since one day. No present or past history of convulsion or any significant birth history was noted. There was a history of progressive regression of developmental milestones in the form of forgetting the ability to sit, read, walk, recognise since last 5 to 6 months. Clinical examination revealed evidence of generalised dystonia, with normal deep tendon reflexes and no evidence of any other abnormality on general or systemic examination except for papilledema. The patient’s haemogram profile revealed haemoglobin of 12.60 gm/dL, and normal leukocyte count (10,000/cmm). Her liver function tests were within normal limits (ALT=11 IU/L, AST=28 IU/L) with a normal ceruloplasmin level (35.76 mg/dL). Her MRI brain scan revealed abnormal signal intensities appearing hypointense on T1 weighted images [Table/Fig-1a,b] and hyperintense on T2 weighted [Table/Fig-2a,b] and FLAIR images [Table/Fig-2c] involving bilateral caudate nuclei, lentiform nuclei, peri-trigonal and subcortical white matter in bilateral frontal-parietal and left occipital lobes. The signal intensities in lentiform and caudate nuclei also showed hyperintensities on DWI with corresponding signal drop on Apparent Diffusion Coefficient (ADC) maps [Table/Fig-3a,b] with no evidence of any blooming on Susceptibility Weighted Imaging (SWI) [Table/Fig-1c]. There was no evidence of abnormal post contrast enhancement [Table/Fig-3c]. Considering the age of the patient and imaging features we put forward a differential diagnosis of acute metabolic encephalopathy or late presentation of wilson’s disease. However, owing to normal ceruloplasmin and normal biochemical parameters, these conditions were ruled out. EEG was done in this patient which showed generalised rapid sharp wave discharges [Table/Fig-4] and hence the diagnosis of NCSE was made. The child was managed on aggressive antiepileptic and supportive therapy.
Axial T1 weighted MR images show abnormally hypointense lentiform and caudate nuclei bilaterally (a,b). No evidence of blooming was noted on SWI images (c).
Axial T2 weighted and FLAIR MR images show abnormal hyperintensities involving bilateral lentiform and caudate nuclei (a,b) as well as white matter hyperintensities in bilateral fronto-parietal and left occipital lobes (b,c).
Axial DWI image (a) shows hyperintensities in basal ganglias which corresponding signal drop on ADC maps (b) with no evidence of post contrast enhancement (c).
Electroencephalogram of our patient showing generalised sharp wave spike epileptiform discharges typical of non-convulsive status epilepticus.
Discussion
There is a lack of a consensus definition on NCSE. It is generally defined as any prolonged seizure activity lasting for more than 30 minutes but with the absence of classic tonic clonic convulsive movements typical of seizures. There are two main types of NCSE: Absence or complex partial status epilepticus (ASE or CPSE) characterised by confused or abnormal behaviour and Subtle status epilepticus (SSE) manifesting as facial or hand twitchings particularly in comatose patients [1]. Canas N et al., reported that DWI (with ADC map), is the most sensitive MRI technique to evaluate transient changes of periictal state. Hyperintensities are detected on DWI and corresponding signal drop in ADC values is noted as in our case, which is maximum between 30-90 minutes correlating with neuronal loss in the post ictal phase [2]. However, as reported by Lansberg MG et al., the abnormalities are seen classically in cortical-subcortical locations or less commonly only cortical or only subcortical [2-4]. The authors had hypothesised a cerebellar diaschisis phenomenon and abundant synaptic connections between cerebellum and brain stem as a cause for this contagious involvement.
Hypointensity on T1-Weighted Images (WI) and hyperintensity on T2-WI and Fluid Attenuation Inversion Recovery (FLAIR) without any evidence of post contrast enhancement can be seen on conventional MR sequences as in our case. Canas N et al., reported that status epilepticus may produce a brain pseudotumoral appearance depicted by a reversible regional enhancement in postcontrast T1-WI owing to blood brain barrier disruption [5]. No cerebellar involvement was noted in our case nor was any enhancement seen. All these findings together with clinical evidence of regression of milestones and amnesia led us believe this to be a manifestation of metabolic encephalopathy. Localised areas of hyper as well as hypoperfusion may be seen on contrast based perfusion-MR using arterial spin labeling [6,7]. SWI in our case did not reveal any abnormalities. However, as reported by Gasparotti R et al., and Aellen J et al., focal areas of pseudonarrowing or pseudodiminishing of cortical veins may be seen and help differentiate stroke and NCSE [8,9]. Altered metabolite ratios may be seen in the form of decreased N-acetylaspartate (NAA) and/or lactate peak or decreased NAA to Creatine (Cr) ratio and increased glutamine/Cr and decreased glutamate on MR spectroscopy [10,11]. Refractory status epilepticus may show bilateral claustrum T2/FLAIR and DWI hyperintensity with normal ADC maps [12]. The DWI signal abnormalities in our case were predominantly in deep white matter, basal ganglia and thalamus. Cartagena AM et al., reported signal abnormalities on DWI involving basal ganglia, thalamus, brainstem, cerebellum and external capsules in 9 out of 10 cases thus supporting our findings of periictal changes on DWI [13].
Treatment of NCSE depends on the underlying cause and precise syndromic diagnosis. Initial control of seizures may be achieved using buccal midazolam or intravenous lorazepam. Atypical absence may not respond to benzodiazepines and hence emergency medication like phenobarbitone may be required. Typical absence seizure precipitated by inappropriate antiepileptic drugs may need discontinuation of the medication [14].
Conclusion
Absence of visible seizure manifestations may lead clinician in the intensive care unit disregard the diagnosis of NCSE making it a diagnostic imaging challenge. In children it can present with altered mental status and change in behaviour as many other metabolic disorders affecting myelination and further mental development. MR imaging findings are very non specific and hence may require first to rule out more sinister differential diagnoses like wilson’s disease and leigh’s disease which may present with similar involvement of basal ganglias and deep white matter as in our case. Serum abnormalities and EEG can be helpful in ruling out the other major differential diagnoses. Hence, interpreting radiologist should always consider NCSE as a differential while interpreting MR scans of paediatric child with developmental abnormalities to minimize management delays.
[1]. Andrew KC, Shlomo S, Non convulsive status epilepticusEmerg Med Clin N Am 2011 29:65-72.10.1016/j.emc.2010.08.00621109103 [Google Scholar] [CrossRef] [PubMed]
[2]. Canas N, Breia P, Soares P, Saraiva P, Calado S, Jordao C, The electroclinical-imagiological spectrum and long-term outcome of transient periictal MRI abnormalitiesEpilepsy Res 2010 91(2-3):240-52.10.1016/j.eplepsyres.2010.07.01920728314 [Google Scholar] [CrossRef] [PubMed]
[3]. Lansberg MG, O’Brien MW, Norbash AM, Moseley ME, Morrell M, Albers GW, MRI abnormalities associated with partial status epilepticusNeurology 1999 52:1021-27.10.1212/WNL.52.5.102110102423 [Google Scholar] [CrossRef] [PubMed]
[4]. Kim JA, Chung JI, Yoon PH, Kim DI, Chung TS, Kim EJ, Transient MR signal changes in patients with generalised tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imagingAm J Neuroradiol 2001 22:1149-60. [Google Scholar]
[5]. Canas N, Soares P, Calado S, Pestana R, Ribeiro C, Vale J, Pathophysiology and long term outcome of reversible tumor -like lesions induced by presenting status epilepticusJ Neuroimaging 2010 20:169-74.10.1111/j.1552-6569.2008.00334.x19453829 [Google Scholar] [CrossRef] [PubMed]
[6]. Flacke S, Wuellner U, Keller E, Hamzei F, Urbach H, Reversible changes in echoplanar perfusion- and diffusion weighted MRI in status epilepticusNeuroradiology 2000 42:92-95.10.1007/s00234005002110663481 [Google Scholar] [CrossRef] [PubMed]
[7]. Calistri V, Caramia F, Bianco F, Fattapposta F, Pauri F, Bozzao L, Visualization of evolving status epilepticus with diffusion and perfusion MR imagingAm J Neuroradiol 2003 24:671-73. [Google Scholar]
[8]. Gasparotti R, Pinelli L, Liserre R, New MR sequences in daily practice: Susceptibility weighted imaging. A pictorial essayInsights Imaging 2011 2(3):335-47.10.1007/s13244-011-0086-322347957 [Google Scholar] [CrossRef] [PubMed]
[9]. Aellen J, Abela E, Buerki SE, Kottke R, Springer E, Schindler K, Focal hemodynamic patterns of status epilepticus detected by susceptibility weighted imaging (SWI)European Radiology 2014 24(11):2980-88.10.1007/s00330-014-3284-925097124 [Google Scholar] [CrossRef] [PubMed]
[10]. Wu Y, Pearce PS, Rapuano A, Hitchens TK, de Lanerolle NC, Pan JW, Metabolic changes in early post status epilepticus measured by MR spectroscopy in ratsJ Cereb Blood Flow Metab 2015 35(11):1862-70.10.1038/jcbfm.2015.14526104287 [Google Scholar] [CrossRef] [PubMed]
[11]. Zahr NM, Crawford EL, Hsu O, Vinco S, Mayer D, Rohlfing T, In vivo glutamate decline associated with kainic acid induced status epilepticusBrain Res 2009 1300:65-78.10.1016/j.brainres.2009.08.06019715683 [Google Scholar] [CrossRef] [PubMed]
[12]. Meletti S, Slonkova J, Mareckova I, Monti G, Specchio N, Hon P, Claustrum damage and refractory status epilepticus following febrile illnessNeurology 2015 85(14):1224-32.10.1212/WNL.000000000000199626341869 [Google Scholar] [CrossRef] [PubMed]
[13]. Cartagena AM, Young GB, Lee DH, Mirsattari SM, Reversible and irreversible cranial MRI findings associated with status epilepticusEpilepsy Behav 2014 33:24-30.10.1016/j.yebeh.2014.02.00324614522 [Google Scholar] [CrossRef] [PubMed]
[14]. Adiga R, Non convulsive status epilepticus in childrenAdvances in Clinical Neuroscience and Rehabilitation 2012 12(1):14-15. [Google Scholar]