Diabetes mellitus is one of the most concerning metabolic illnesses with long term complications resulting in disability and death, with type 1 occurring mostly in children and young adults and type 2 in adults. For type 2 diabetes, in addition to treatments with insulin or oral hypoglycaemic agents, life-style modification is effective in assisting diabetes patients to reduce their blood glucose or A1C levels [1,2]. Behavioural intervention and self-management education have been proved to enhance diabetes control [3-5]. Additionally, providing diabetes education in a group setting is more effective than an individual education course [6]. Therefore, many strategies have been implemented to improve diabetes education via various behavioural group interventions for type 2 diabetes patient [1,3-5], including diabetes camp [7-10]. For type-1 diabetes with insulin therapy, diabetes self-management is a crucial part of treatment in children. One popular behavioural group intervention is a diabetes camp which is an educational activity where patients are provided with information about diabetes through various fun learning activities. Most data indicates that a short-term residential diabetes camp provides several advantages to the patients. A camp over a 1-week period can significantly improve the patients’ knowledge and awareness of the need for diabetes self-management [11,12]. One study has also shown that following attendance at a 2-week diabetes camp, the blood glucose levels of the patients were reduced, based on the analysis of the level of fructosamine. Additionally, blood analysis during the 3rd and 7th month following the camp continued to show the reduction of the A1C values against the control [13].
In spite of providing several advantages to diabetes children, there has been limited information on diabetes camp for adults with type 2 diabetes [7-10]. Due to their individual responsibilities, some adult type 2 diabetes patients may not be able to attend a camp of more than 1 day duration. Therefore, in Thailand, a strategy of a diabetes camp without staying overnight, as an alternative, is commonly practiced. A 1-day diabetes group education session, commencing in the morning and finishing in the afternoon, or a so-called ‘Diabetes Day Camp’, is a popular strategy to cope with type 2 diabetes with uncontrollable blood glucose levels. Educational information is provided through fun learning activities resembling a diabetes camp except for duration differences. From author’s observations, many so-called diabetes day camps have been conducted for adult type 2 diabetes each year by provincial and community hospitals. In 2017 alone, all community hospitals in our province reported organising this activity. Most of the activities have been conducted as a stand-alone event. However, it might be questioned whether these 1-day diabetes education practice are based on feasibility rather than evidence-based studies and, therefore, might be insufficient in terms of duration. For the very short-term behavioural intervention, there was some discordant information on the effectiveness in terms of glycaemic control in type 2 diabetes. To the best of author’s knowledge, there has been only two Randomised Controlled Trials (RCT) studying the 1-day diabetes education session. One study from the UK comparing glycaemic control between the intervention group participating in a 6-hour education session over one day or two half day periods and the control, found no significant differences in A1C values [14]. The other study conducted with Thai type 2 diabetes patients randomly assigned the patients to attend a so-called 1-day diabetes day camp found improvement in their knowledge of diabetes self-management, and their fasting blood glucose levels significantly decreased [8].
Some investigators may argue against the efficacy of a 1-day camp to expect any changes in glycaemic control and prefer more tangible outcomes such as retention of knowledge. However, since improvement in diabetes self-management has already been expected and given that the ‘Diabetes Day Camp’ is a common practice in Thailand, it could be worth measuring the outcome in terms of glycaemic control to document the effectiveness of this group education practice. In author’s hypothesis, participants who attended the education session might behave better in controlling their food consumption, at least for a short period of time after the intervention, and then might return to their earlier behaviour. Fructosamine levels, which were used to determine patients’ accumulated blood glucose in a 2-3 weeks period earlier, might reflect their improvement shortly after finishing the session more efficiently than A1C levels. Therefore, this study chose to assess the outcome by these two measures: fructosamine values for short-term and A1C levels for longer-period evaluation. Given that A1C levels are a key determinant of death rate and complication risk [15], the aim of the current study was, therefore, to investigate the effectiveness of a 1-day diabetes group education session or a so-called ‘Diabetes Day Camp’ in reducing A1C levels, as well as fructosamine levels, in type 2 diabetes patients.
Materials and Methods
A Randomised Control Trial was conducted between May 2015 and January 2016 at the Diabetes Clinic, Naresuan University Hospital and 6 health promotion hospitals in the health network centered at Naresuan University Hospital. Type 2 diabetes patients aged 30-80 years with A1C levels exceeding 8% and who were receiving insulin treatment exceeding 10 units per day with or without oral hypoglycaemic agent were recruited. The study was designed to have 70 patients randomly assigned to either the group who would attend a so-called ‘Diabetes Day Camp’, or to the non-participating control, 35 to each group. Assignment was conducted by computerised block randomisation, 1:1 allocation. The allocation sequence was kept in an envelope by one of our study team not involve with the patient enrollment. The sequence was disclosed one by one when intervention was to be assigned to the enrolled patients (among the authors, PW generated the random allocation sequence, SS enrolled participants, and PB assigned patients to interventions). Patients were excluded from the study for non-compliance with recommended treatment regimes, being on steroid treatment or needing insulin/oral hypoglycaemic agent adjustment 1 month before and 3 months after finishing the camp. Patients with incomplete results were also excluded.
The so-called 1-day ‘Diabetes Day Camp’ was held between 8.00 am and 3.00 pm at the out-patient clinic of the hospital, and two sessions were scheduled, both of which were identical in the activities and personnel involved. Patients could make a choice to attend one of the two sessions at their convenience. Clinical evaluation of all participating patients undertaken before entering the camp included: demographic data, duration of diabetes condition, diabetes complications, medication, nutritional status, weight and height, blood pressure, problems of insulin injection site and technique, diabetes behaviour, and quality of life. At both camps, each patient received a food card indicating their specific energy requirements and the calculated proportions of food servings in their diet. Each patient had a capillary blood glucose performed before breakfast and lunch, and two hours after breakfast and lunch on the day of the camp. Patients attending the camp learned together in groups of 5 or 6. Patients were provided with information through various fun learning activities. The information provided included: general knowledge about diabetes, diet for diabetes, foot care in diabetes, exercise appropriate for diabetes patients, and hypoglycaemic treatment and prevention. Patients also received information on self-monitoring of blood glucose interpretation and correct practices for low or high blood glucose situations. All camp staff (endocrinologists, pharmacists, nurses, nutritionists) had more than five years’ experience in setting up a diabetes camp. All attendees at the camp were tested for their knowledge of diabetes and associated matters before and after attending the camp. For the non-participating control, they were followed and treated on a regular basis in parallel to the interventional group. Their glycaemic outcomes were determined at the time point according to the intervention group.
The effectiveness of the camp as the primary outcome was determined by analysing glycaemic control of the patients, demonstrated by testing of their serum fructosamine levels (ARCHITECTc8000 analyser, Abbott Laboratories, Illinois, USA) at 2 weeks and A1C levels (D-10 haemoglobin analyser, Bio-Rad Laboratories, California, USA) at 3 months after attending the camp together with their baseline values. Secondary outcomes were lipid profile (cholesterol level, triglyceride level, HDL level, and LDL level), changes of body weight, and quality of life, as determined by SF-36 [16]. All parameters were evaluated 3 months after finishing the camp. The study was approved by the Institutional Ethics Committee of Naresuan University (Institutional Review Board number 368/57). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all patients before entering the study.
The final sample size of 70 patients, 35 in each group, was calculated to be sufficient to provide a 90% probability of detecting a 1% decrease of A1C between the groups. We chose the difference of A1C values of 1%, based on experience from author’s before and after analysis within group in our previous 1-day Diabetes Day Camp.
Statistical Analysis
All data analyses were performed using the SPSS 12.0 software package. Chi-square test and independent t-test were used for comparison of demographic data and final outcomes between the experimental group and the control. Before and after outcome analysis within group was determined by paired t-test.
Results
The study included a total of 84 adult diabetes patients. After randomisation, 42 were assigned to attend a so-called ‘Diabetes Day Camp’ and 42 allocated to the control group. The two consecutive camps were held on August 8, 2015, with 31 participants, and on October 31, 2015, with 11 attendees. Of the 84 patients included, 5 did not subsequently attend the camp after assignment, a further 9 were lost in the follow-up period, and 1 patient died due to cancer. All of these patients were excluded from the study. The numbers of fully participating patients were, therefore, 33 and 35 in the experimental and control group, respectively [Table/Fig-1].
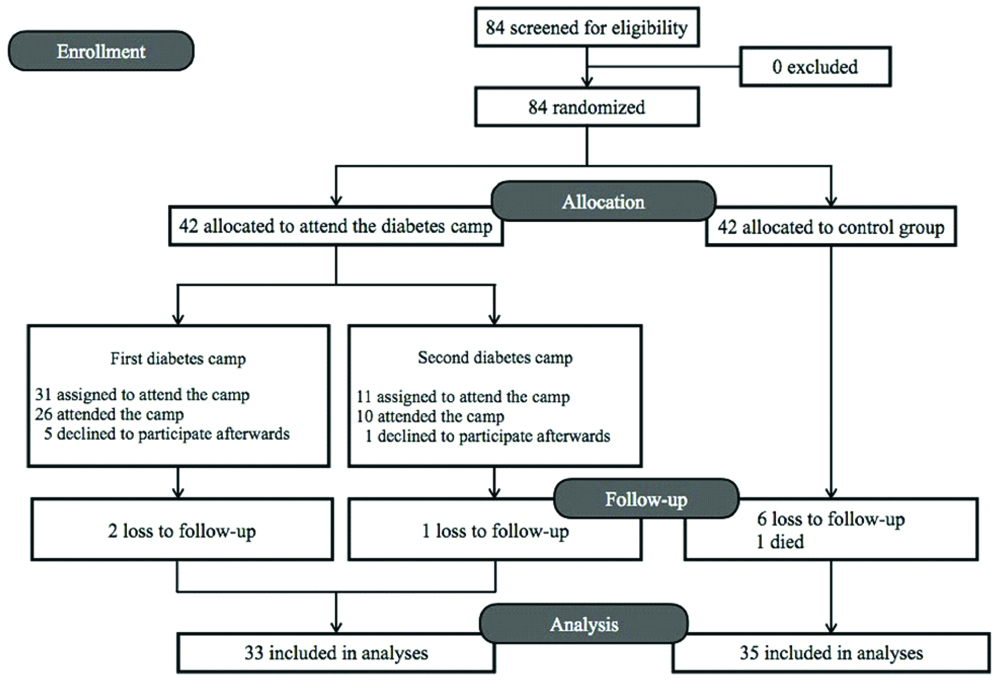
There were no significant differences in the baseline demographic data of the patients between the groups [Table/Fig-2]. Even though the proportion of proliferative diabetes retinopathy seemed higher in the camp participants, no statistical differences was noted [Table/Fig-3]. The mean pre- and post-test scores for the level of knowledge on diabetes, diet, and treatments in the camp participants were 19.43±3.16 and 21.87±2.94, respectively (p<0.001).
Demographic data of the patients between the two groups at baseline.
| Intervention group (N=33) | Control group (N=35) | p-value |
|---|
| Age (year) | 57.4±9.9 | 54.9±12.4 | 0.360† |
| Male gender (%) | 12 (36.4) | 13 (37.1) | 0.947‡ |
| Education level (%) |
| Primary school | 20 (60.6) | 18 (51.4) | 0.790‡ |
| Secondary school | 6 (18.2) | 6 (17.1) |
| Bachelor | 5 (15.2) | 7 (20.0) |
| No education | 2 (6.1) | 4 (11.4) |
| Occupation (%) |
| Farmer | 9 (27.3) | 9 (25.7) | 0.448‡ |
| Merchant | 8 (24.2) | 6 (17.1) |
| Labourer | 4 (12.1) | 5 (14.3) |
| Government official or State enterprise | 2 (6.1) | 7 (20.0) |
| Others | 2 (6.1) | 3 (8.6) |
| Not working | 8 (24.2) | 5 (14.3) |
| Income (per month) (%) |
| <5,000 baht | 7 (21.2) | 10 (28.6) | 0.192‡ |
| 5,000-10,000 baht | 14 (42.4) | 10 (28.6) |
| 10,000-20,000 baht | 9 (27.3) | 6 (17.1) |
| >20,000 baht | 3 (9.1) | 9 (25.7) |
†p-value comparing between the two groups using unpaired t-test
‡p-value comparing between the two groups using Chi-square test
Characteristics of diabetes between the two groups at baseline.
| Intervention group (N=33) | Control group (N=35) | p-value |
|---|
| Duration of diabetes (year) | 4.2±1.2 | 4.2±1.0 | 0.795† |
| Medication (%) |
| Insulin with OHAs | 26 (78.8) | 29 (82.9) | 0.670‡ |
| Insulin only | 7 (21.2) | 6 (17.1) |
| Diabetic complication | | | |
| Diabetic nephropathy (%) |
| Microalbuminuria | 10 (30.3) | 11 (32.4) | 0.801‡ |
| Macroalbuminuria | 8 (24.2) | 6 (17.6) |
| Normoalbuminuria | 15 (45.5) | 17 (50.0) |
| Diabetic retinopathy (%) |
| Mild NPDR | 3 (9.1) | 5 (15.6) | 0.366‡ |
| Moderate NPDR | 1 (3.0) | 1 (3.1) |
| Severe NPDR | - | - |
| PDR | 5 (15.2) | 1 (3.1) |
| No diabetic retinopathy | 24 (72.7) | 25 (78.1) |
| Coronary artery disease (N) | - | 2 | |
| Diabetic neuropathy (N) | 3 | - | |
†p-value comparing between the two groups using unpaired t-test
‡p-value comparing between the two groups using Chi-square test
OHA: Oral hypoglycaemic agent; PDR: Proliferative diabetic retinopathy; NPDR: Non-proliferative diabetic retinopathy
Comparing glycaemic control between the camp participants and the control, no significant differences in serum fructosamine and A1C values were found. At 2 weeks after finishing the camp, the mean (95% confidence interval) fructosamine value for the camp participants was 281.03±49.20 mg/dL (263.59-298.48 mg/dL) as against 287.94±74.08 mg/dL (262.49-313.39 mg/dL) for the control (p=0.654). At 3 months after attending the camp, the mean A1C values for the camp participants and the control were 8.99±1.37% (8.51-9.48%) and 9.16±2.03% (8.46-9.86%), respectively (p=0.695) [Table/Fig-4].
Comparison of fructosamine and A1C levels between and within the group.
| Glycaemic control | Intervention group (N=33) | Control group (N=35) | p-value† |
|---|
| Fructosamine level at baseline (mg/dL) | 291.75±56.25 | 296.91±77.00 | 0.757 |
| Fructosamine level 2 weeks after (mg/dL) | 281.03±49.20 | 287.94±74.08 | 0.654 |
| p-value‡ | 0.138 | 0.131 | |
| A1C level at baseline (%) | 9.50±1.37 | 9.54±1.81 | 0.913 |
| A1C level 3 months after (%) | 8.99±1.37 | 9.16±2.03 | 0.695 |
| p-value‡ | 0.014 | 0.074 | |
†p-value comparing between the two groups using unpaired t-test
‡p-value comparing between before and after outcomes within group using paired t-test
There was no significant reduction in the levels of serum fructosamine before and after attending the camp. The mean fructosamine levels at baseline and 2 weeks after, in the camp participant group were 291.75±56.25 mg/dL and 281.03±49.20 mg/dL, respectively (p=0.138), and in the control group were 296.91±77.00 mg/dL and 287.94±74.08 mg/dL, respectively (p=0.131). However, there was a significant reduction in A1C levels in the camp participants’ group (9.50±1.37% at baseline versus 8.99±1.37% at 3 months after, p=0.014), whereas there were no significant differences in the control group (9.54±1.81% at baseline versus 9.16±2.03% at 3 months after, p=0.074) [Table/Fig-4].
As a secondary outcome, there was a significant difference in the mean cholesterol, LDL, and HDL levels of the camp participant group compared to the control group at 3 months after attending the camp. The mean cholesterol, LDL, and HDL levels for the camp participant group were 172.94±36.57 mg/dL, 94.05±33.93 mg/dL, and 50.94±11.14 mg/dL, respectively (177.55±35.85 mg/dL, 99.55±28.79 mg/dL, and 51.30±9.11 mg/dL, respectively at baseline) as compared to the control group’s readings of 207.60±62.42 mg/dL, 120.97±44.54 mg/dL, and 45.09±10.10 mg/dL, respectively (198.17±56.95 mg/dL, 117.40±49.46 mg/dL, and 47.23±10.04 mg/dL, respectively at baseline) (p=0.007, 0.007, and 0.026, respectively) [Table/Fig-5]. However, there were no significant differences between the camp participant group and the control group at 3 months after attending the camp in triglyceride levels (135.21±96.36 mg/dL versus 175.54±110.25 mg/dL, respectively, p=0.119) and change of body weight (-0.33±2.03 kg versus -1.1±1.99 kg, respectively, p=0.119) [Table/Fig-5]. In addition, there was no improvement in quality of life between the two groups at 3 months after attending the camp [Table/Fig-6].
Comparison of lipid profile and body weight change between the camp participants group and the control before and after attending the camp.
| Baseline | p-value† | 3 months | p-value† |
|---|
| Intervention | Control | Intervention | Control |
|---|
| Cholesterol (mg/dL) | 177.55±35.85 | 198.17±56.95 | 0.081 | 172.94±36.57 | 207.60±62.42 | 0.007 |
| Triglyceride (mg/dL) | 137.00±86.43 | 179.77±92.33 | 0.053 | 135.21±96.36 | 175.54±110.25 | 0.119 |
| HDL-Cholesterol (mg/dL) | 51.30±9.11 | 47.23±10.04 | 0.085 | 50.94±11.14 | 45.09±10.10 | 0.026 |
| LDL-Cholesterol (mg/dL) | 99.55±28.79 | 117.40±49.46 | 0.076 | 94.05±33.93 | 120.97±44.54 | 0.007 |
| BW change (kg) | | | | -0.33±2.03 | -1.10±1.99 | 0.119 |
†p-value comparing between the two groups using unpaired t-test
HDL: High-density lipoprotein; LDL: Low-density lipoprotein; BW: Body weight
Comparison of quality of life score between the camp participants and the control at baseline and after 3 months.
| Quality of life dimension | Baseline | p-value† | 3 months | p-value† |
|---|
| Intervention | Control | Intervention | Control |
|---|
| Physical dimension | 72.64±23.12 | 68.89±24.18 | 0.52 | 70.47±24.70 | 69.25±22.80 | 0.835 |
| Health problem dimension | 60.16±40.09 | 67.86±39.56 | 0.432 | 66.41±43.35 | 54.29±43.92 | 0.260 |
| Illness dimension | 63.58±20.02 | 69.50±21.16 | 0.245 | 65.00±19.64 | 62.21±23.78 | 0.605 |
| Overall QOLs dimension | 46.25±25.11 | 53.21±25.63 | 0.266 | 49.38±16.64 | 54.51±18.23 | 0.234 |
| Vivacity dimension | 53.98±24.36 | 56.31±24.17 | 0.695 | 59.69±16.50 | 57.67±19.13 | 0.646 |
| Social dimension | 69.17±28.37 | 75.36±31.58 | 0.404 | 75.00±22.20 | 84.64±26.44 | 0.113 |
| Dimension of limitation in mind | 59.38±41.25 | 65.71±45.36 | 0.553 | 74.48±33.59 | 65.71±42.38 | 0.355 |
| Mental health dimension | 70.38±21.06 | 73.06±23.38 | 0.625 | 74.00±18.17 | 77.69±18.39 | 0.413 |
| Average of QOLs | 61.25±22.15 | 67.68±21.34 | 0.224 | 65.21±20.92 | 66.04±18.99 | 0.847 |
†p-value comparing between the two groups using unpaired t-test
QOL: Quality of life
Discussion
The so-called 1-day Diabetes Day Camp organised by the Diabetes Clinic of Naresuan University Hospital and 6 Health Promotion Hospitals did not improve glycaemic control in the type 2 diabetes patients when compared with the usual care offered to the control. Despite before and after analysis within group of the patients who participated in the camp demonstrating significant improvement in their A1C values and levels of knowledge, between-group analysis with the control conversely identified no differences in glycaemic control. The results of the present study add on substantial evidence regarding of the effectiveness of the 1-day ‘Diabetes Day Camp’ on glycaemic control of type 2 diabetes. This finding was similar to the results of the RCT from the UK except for a minimal duration difference and a distinct mode of education delivery in conducting the education session [14]. Additionally, despite the positive result from the other previous RCT [8] comparing glycaemic control using fasting blood glucose level 1 month after attending the camp in type 2 diabetes, the determinants for glycaemic control were not comparable to present study.
Behavioural intervention and self-management education have been proved to be an essential tool for improving clinical outcomes in adult type 2 diabetes patients [3-5]. However, type of intervention and mode of education delivery are important issues to be considered. Apart from the two discordant researches on a 1-day diabetes education session mentioned earlier [8,14], most of the limited studies investigating the use of a diabetes camp for adult type 2 diabetes also supported the benefits of the activity [7,9,10]. Two strategies in common in these three supporting studies which differed from present study ‘Diabetes Day Camp’ comprised of extending camp duration [7] and adding some booster camps [9,10]. It may be that 1-day ‘Diabetes Day Camp’ is too short in duration and is not sufficient to change participants’ ongoing behaviours to improve glycaemic control. In addition to a very-short-term behavioural intervention like ours, there were plenty of RCTs of short-term interventions with booster and longer-duration activities for meta-analysis which proved to be effective for improving glycaemic control for adults with type 2 diabetes [4,5]. Since the ‘Diabetes Day Camp’ is a common practice in Thailand, therefore, for a cost-effectiveness reason, if the effectiveness could not be proved apparently, this very short-term intervention should not be conducted as a stand-alone activity at this present time, but should be incorporated into a set of continuing education programs for diabetes self-management.
Regarding the 6 subjects excluded from the interventional group based on unwillingness to attend the camp after randomisation, since there were no participants excluded from the control due to the same reason, this could diminish the benefit of randomisation. Therefore, we did a re-analysis with these 6 subjects’ data included, which showed the same results. At 3 months after attending the camp, the mean A1C values for the camp participants with these 6 subjects’ data included (n=39) and the control (n=35) were 8.78±1.40% and 9.16±2.03%, respectively (p=0.355). There were no data of fructosamine value from these 6 subjects for re-analysis.
According to author’s initial hypothesis by utilising fructosamine values for short-term and A1C levels for longer-period evaluation, after analysis, we could not demonstrate any discordance between fructosamine and A1C levels in each subject. In the intervention group, these correlation values (r2) between the two measures were almost the same at the beginning (0.498, p=0.004) and the end (0.518, p=0.002) of the education session. The same correlation also occurred in the control. With the homogeneous concordance results, we could not identify any participants who might be or might not be benefiting from intervention.
As a secondary outcome, patients’ lipid profiles were improved after finishing the intervention. Despite the lipid values of the control seeming to be higher when compared with the camp participants’ levels at the beginning of the camp, the difference was more pronounced after finishing the intervention. However, since we did not exclude patients with any adjustment of lipid lowering drugs before and after finishing the camp, it may not be justified to conclude that this outcome was a positive result.
Limitation
The limitations of study include the small sample size and the design of the study to eradicate any possible confounders. As we chose to calculate the sample size from the difference of A1C values of 1%, however, the difference between groups may be as narrow as 0.17% in absolute value, as is the result of this current study. Therefore, there was no statistically significant difference in A1C values identified between the groups, despite the positive result from the before and after analysis within the camp participant group. Two consecutive sessions of camp may be another possible confounder. Since numbers of the two camps’ attendees are quite different, this could make the experience different for the subjects and could introduce additional variability to present data. However, due to adult patients’ availability, we need to organise more than one event to recruit a sufficient number of subjects to the study. Regarding the main confounding factor which can affect glycaemic control besides intervention is the adjustment of insulin or oral hypoglycaemic agents by the physician. To avoid this influence, we chose to exclude patients with drug adjustment within 1 month before and 3 months after finishing the camp before attaining the outcomes. Therefore, longer-period evaluation seems impracticable. However, regardless of this confounder, we did an additional study by extending the analysis on A1C of the remaining participants at one year after finishing the camp. Additional results comparing glycaemic control between the camp participants and the control over a longer duration after finishing the intervention also revealed insignificant differences in A1C values. Over a one year period after attending the camp, the mean A1C values for the remaining camp participants and the control were 9.23±1.81% (n=32) and 9.27±1.94% (n=33), respectively (p=0.929), resembling the main results. Finally, regarding the practicability aspect of the health education program implemented, it may be difficult to compare the effectiveness of different education programs in clinical practice. However, with present education modules, the camp seems completely practical for covering all the aspects of diabetes control needed.
To apply this finding for other countries and cultures, the disease severity, educational background and socioeconomic status of the population have to be considered. The diabetes participants may be categorised as a hard-to-control group. In the provincial health care setting, the majority of the patients were farmers and employees with somewhat low education level and income. These patients’ characteristics may be similar to other developing countries’ population, especially in the South East Asia. Regarding the cost in conducting education sessions, each participant’s expense was 38 US dollars on average (for capillary blood glucose monitoring, food, and traveling expenses), which is concordant to the low cost of living in the region.
Conclusion
The program of behavioural modification through a 1-day diabetes group education session in type 2 diabetes patients could not be shown to improve glycaemic control compared with non-intervention. However, in light of the positive result from A1C levels before and after analysis and improvement in knowledge of diabetes self-management within the interventional group, it seems that the program could be beneficial, especially if further modified. Further studies should be considered to evaluate the effectiveness of longer-duration camps, or, alternatively, a 1-day camp with booster interventions. Additionally, the results should be incorporated in an analysis for a cost-effectiveness of this common educational activity in the country.
†p-value comparing between the two groups using unpaired t-test
‡p-value comparing between the two groups using Chi-square test
†p-value comparing between the two groups using unpaired t-test
‡p-value comparing between the two groups using Chi-square test
OHA: Oral hypoglycaemic agent; PDR: Proliferative diabetic retinopathy; NPDR: Non-proliferative diabetic retinopathy
†p-value comparing between the two groups using unpaired t-test
‡p-value comparing between before and after outcomes within group using paired t-test
†p-value comparing between the two groups using unpaired t-test
HDL: High-density lipoprotein; LDL: Low-density lipoprotein; BW: Body weight
†p-value comparing between the two groups using unpaired t-test
QOL: Quality of life