The upsurge of multidrug resistant tuberculosis has converted curable tuberculosis into a deadly disease [1]. WHO in its guidelines has endorsed that people with TB resistant to rifampicin, with or without resistance to other drugs, should be treated with an MDR-TB treatment regimen [2]. Thus, rifampicin can be targeted for early detection of MDR-TB. Introduction of molecular method has created a renaissance in this direction but methods that are simple, rapid and economical are still required to fill the gaps in the areas where molecular techniques are not available.
Colorimetric methods have yielded encouraging results for rapid detection of drug resistance [3-8]. Some authors have used 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay and have found it to be less expensive, useful and rapid and have recommended them for use with ease in the developing countries [9-12]. In developing countries we have an ideal situation for the utility of such tests. MTT, a yellow coloured dye in its oxidised form is reduced in the presence of dehydrogenase enzyme in metabolically active cells, to purple coloured formazan, which can be seen and read both visually and by the colorimeter or spectrophotometer [11].
In 2008, Indirect colorimetric MTT (IMTT) test was evaluated, established and reported in our laboratory [13]. The present study is an extension of the same and has been taken up to evaluate the efficacy of the test for detecting the sensitivity to rifampicin directly on the sputum samples reported positive for AFB. The TB control programs in most countries are still dependant on smear microscopy for detection of Mycobacterium tuberculosis (M. tuberculosis) and there is no report on evaluation of the test against the various grades of smear positivity. The literature available has not described the exact standardisation of DMTT [6-11]. Abate G et al., in their direct test have used their earlier experience with indirect MTT based on bacterial counts, for conducting and evaluating the direct test [6,14,15]. Therefore, in order to make the test more feasible and robust the study was taken up with the objectives to standardise the test against various grades of sputum positivity for AFB, and then evaluate it on clinical samples by comparing the results with the standard Indirect Agar Proportion Method (IAPM) on 7H10 agar, taken as reference standard for both the exercises [16].
This Direct MTT test (DMTT), by eliminating the first step of isolating the organism from the sample, could serve as a step forward in the direction of curtailing the TAT for reporting the drug resistance to rifampicin.
Materials and Methods
The present cross-sectional study duly approved by Institutional Ethics Committee and with written consent from all the patients, was conducted on the sputum samples received in the Department of Microbiology of the Mahatma Gandhi Institute of Medical Sciences, Sevagram, Wardha, Maharashtra, India, between January 2010 to September 2011.
The study was done in three steps:
Firstly, Standardisation of DMTT test was carried out by using 36 AFB positive sputum samples.
Secondly, after standardisation, the DMTT was done on 153 clinical samples.
Lastly, an extended study was taken to standardise the optimum volume of sputum required to obtain good quality interpretable results. This part of the study has not been reported by any of the previous authors.
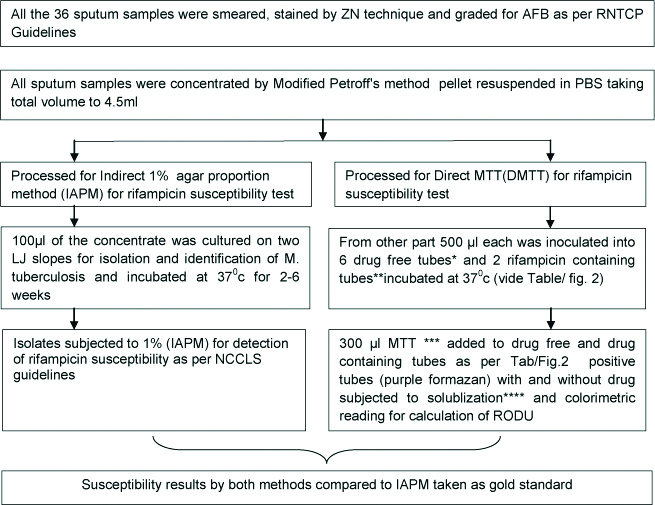
Standardisation of the test was undertaken for which sputum samples positive for AFB, 36 in number, were collected and processed as per the algorithm shown in [Table/Fig-1]. All the sample were subjected to DMTT and IAPM as per Abate G et al., and National Committee for Clinical Laboratory Standards- M24-A, 2003 guidelines respectively [6,16]. Ten AFB negative sputum samples collected from patients clinically suffering from non tuberculous diseases were taken as controls and subjected to similar processing.
Algorithm for processing of the 36 AFB positive sputum samples for standardisation of DMTT assay.
AFB: Acid fast bacilli; ZN: Ziehl neelson; RNTCP: Revised national tuberculosis control programme; LJ: Lowenstein jenson; RODU: Relative optical density unit
*drug free tube: 3 mL 7H9 media with 10% OADC (oleic acid, albumin, dextrose, catalase)+PANTA (polymixin, amphotericin B, nalidixic acid trimethoprim, azlocillin) plus 0.05% glycerol
**drug containing tube: all of the above + 2 μg/mL rifampicin in Dimethyl suphoxide [11].
***MTT: 5 gm MTT in 1mL phosphate buffer saline at pH 7.2 [6,11].
Solublisation buffer: 20% sodium dodecyl sulphate in 50% aqueous solution of dimethylformamide [6,11].
Media and solutions for DMTT test were prepared as per methodology described by Abate G et al., and WoldeMeskel D et al., using all standard reagents from Difco and Sigma laboratories and the quality of the reagents was tested by putting up the Indirect MTT test with standard rifampicin sensitive H37Rv and resistant JR-3 strains, procured from National Repository of Mycobacterial Cultures, Agra, as control [6,11].
Standardisation of DMTT Test
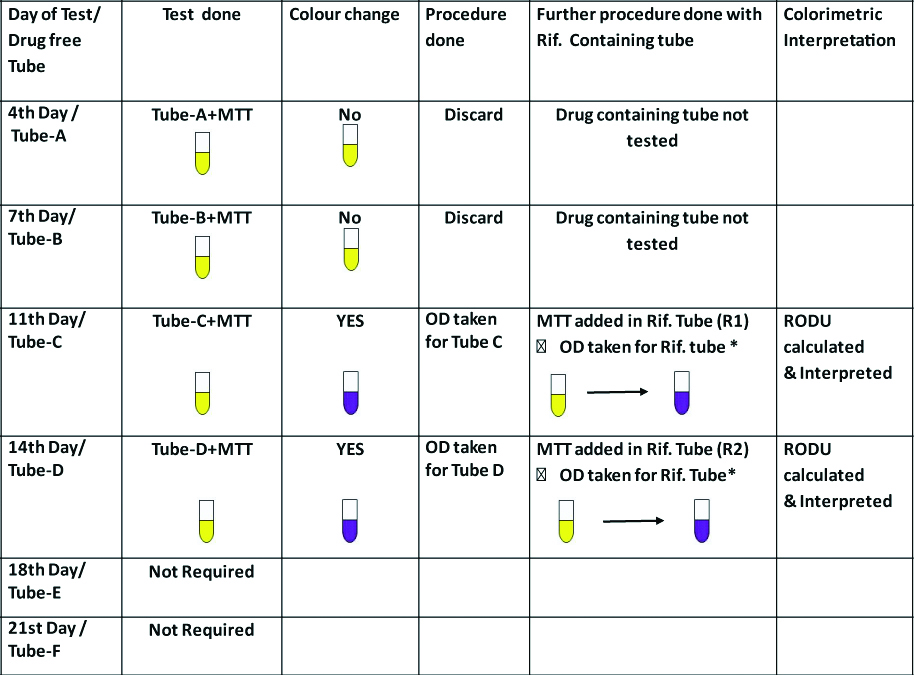
All the 36 positive sputum samples were processed as per the methodology followed by Abate G et al., and as shown in [Table/Fig-2] [6]. All the tubes were incubated and tested, based on our earlier experience with Indirect MTT [13] and other recommendations [6]. The maximum incubation period for the tubes in the present study was restricted to 21 days.
Showing standardisation procedure of Direct MTT assay:
Illustration of DMTT test positive on 11th day.
For standardisation of the DMTT assay total six drug free control tube on 4th (A), 7th (B), 11th (C), 14th (D), 18th (E) and 21st (F) day respectively and two drug containing tubes (R1and R2) were used.
*The tubes showing formazan production were first vortexed and incubated for 4 hours at 37°C and then formazan was dissolved with solubilisation buffer before subjecting to colorimetry.
Calculation for Relative Optical Density Unit (RODU): Optical Density (OD) was measured at 570 nm to standardise the colorimetric reading with a reference control. It was noted as OD=Y for drug free and OD=X for drug containing tube [6,11].

The sample was defined as rifampicin-sensitive when the RODU value was <0.2 and resistant when >0.5. Any value between 0.2 and 0.5 was Borderline Resistance (BR) and Non-interpretable when it was >1 [11]. Each time the test was performed the change in colour by naked eye was also noted and compared with colorimetric readings. If the drug containing tubes did not show any formazan till 21 days the isolate was considered as sensitive.
Quality Check
A day before the MTT test was performed a quality check for contamination, on any of the tubes (drug free or drug containing), was done by sub-culturing the tubes on nutrient agar along with Ziehl Neelson (ZN) and Gram stain. The test was regarded as valid only if Acid Fast Bacilli (AFB) were seen in the tube with no growth on nutrient agar.
DMTT Test on Clinical Samples
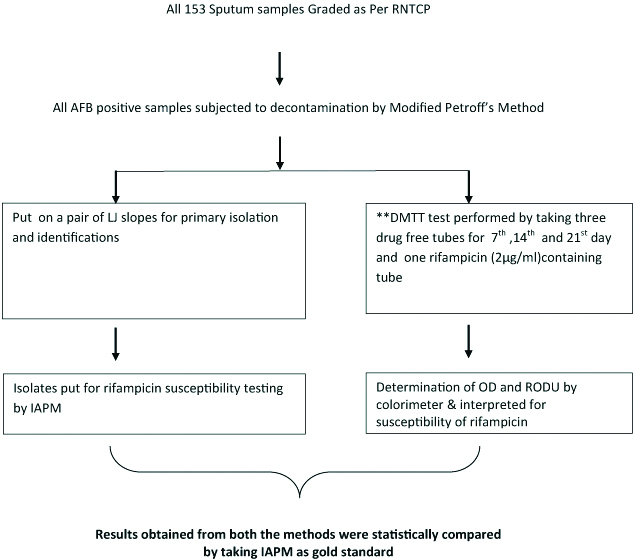
After standardisation, DMTT colorimetric assay was performed on sputum samples received from clinical departments. A total of 153 consecutive AFB positive samples from the patients giving consent and irrespective of the clinical staging of the disease, were included in the study and classified as per the Revised National Tuberculosis Control Program (RNTCP) grading for AFB positive smears. In addition the inclusion criteria were to accept only mucopurutent/purulent sputum with not less than one mL amount and taken with consent of the patient. Exclusion criteria were smear negative sputum, and patient’s not giving consent. The test was performed exactly as described above for standardisation except that, based on the results of standardisation, the test was performed with three drug free tubes and one drug containing tube [Table/Fig-3] instead of six drug free tubes and two drug containing tubes used for standardisation [Table/Fig-2], The results were interpreted and compared with the reference standard IAPM test put up simultaneously for each sample.
Algorithm for processing of clinical samples after standardisation of DMTT.
**For DMTT test of clinical samples only three drug free tube and only one drug containing tube (Total four tubes) were used instead of total eight tubes used for standardisation of the DMTT assay.
Results
Results of Standardisation
It was observed that out of the 36 samples tested, none of the drug free control tubes tested positive for growth on the 4th and the 7th day and all the results for the 2+ and 3+ samples were available by the 14th day while all the 36 samples could be read by the 21st day with 3 non interpretable results in scanty (n=1) and 1+ (n=2) group. The RODU of the midweek tube and end of week tube were almost same. Thus, it was concluded that the test could be safely conducted by restricting the drug free tubes to three for 7th, 14th and 21st days and single drug containing tube to be tested on the day following the day the drug free tube is reported as positive [Table/Fig-4].
Results of standardisation of DMTT assay (n=36).
| Grade of Sputum sample | Total no. of samples | No. of sputum sample with growth positivity in days | Not interpretable (if any, tested till 21st Day) |
|---|
| 4th | 7th | 11th | 14th | 18th | 21st |
|---|
| Day | Day | Day | Day | Day | Day |
|---|
| Scantypositive | 9 | Noresult | Noresult | Noresult | Noresult | 3 | 5 | 1 |
| Grade 1+ | 9 | Noresult | Noresult | 1 | Noresult | 5 | 1 | 2 |
| Grade 2+ | 9 | Noresult | Noresult | 4 | 5 | - | - | nil |
| Grade 3+ | 9 | Noresult | Noresult | 7 | 2 | - | - | nil |
| Total | 36 | Noresult | Noresult | 12 | 7 | 8 | 6 | 3 |
DMTT standardisation test results showed 100% concordance with IAPM and visual readings. No false positives were observed with negative sputum controls and the reagent controls were perfect.
Results for Clinical Samples
Among the 153 samples received and processed as per the RNTCP guidelines, 7.84% were scanty AFB positive, 28.75% were grade 1+, 33.98% grade 2+ and 29.41% were grade 3+ [Table/Fig-5]. Irrespective of the grade interpretable results by DMTT were available for 147 (96.07%) samples with 65.35% results being available by the 14th day and 30% on 21st day. All those with grade scanty were positive only on 21st day (83.33%) with 2 tests remaining “not interpretable”, except for one sample (3+), none was positive on 7th day [Table/Fig-5]. The important observation was that the total rate of contamination was only 1.96% and also the not interpretable results were only 3 (1.96%).
DMTT results with respect to AFB grading of 153 sputum samples.
| Sputum grading with total number tested | No. of sample with day of Direct MTT interpretable results | Grade wise Total no. of interpretable results | Contamination | Not interpretable till 21st Day |
|---|
| 7th Day | 14th Day | 21st Day |
|---|
| Scanty pos | 12 | 0 | 0 | 10 (83.33%) | 10 (83.33) | nil | 2 (16.67%) |
| 1+ | 44 | 0 | 14 (31.81%) | 28 (63.63%) | 42 (95.45%) | 2 (4.54%) | nil |
| 2+ | 52 | 0 | 45 (86.53%) | 5 (9.61%) | 50 (96.15%) | 1 (1.92%) | 1 (1.92%) |
| 3+ | 45 | 1 (2.22%) | 41 (91.11%) | 3 (6.67%) | 45 (100%) | nil | nil |
| Total | 153 | 1 (0.65%) | 100 (65.35%) | 46 (30.06%) | 147 (96.07%) | 3 (1.96%) | 3 (1.96%) |
Interpretable results by IAPM were available for 137 (89.5%) isolates against 147 (96.07%) for DMTT [Table/Fig-6]. It was observed that the results for every grade of sputum positivity were better with DMTT compared to IAPM. Further, during processing, there was loss of only 6 sample (3.92%) with DMTT against 16 (10.45%) with IAPM due to no growth on Löwenstein-Jensen (LJ) medium in 9 (5.88%) samples; contamination in 6 (3.9%) and growth of Non Tuberculosis Mycobacteria in one.
Grade wise comparison of results available by DMTT and IAPM.
| Grade of sputum positivity for AFB | Total no. of Samples | No. of samples available with interpretable results in DMTT | Causes for not consideration in DMTT assay result | No. of sample available with interpretable result in IAPM | Causes for not consideration in IAPM result | Final no. of available interpretable result with both the methods | No. of total eliminated samples from both the methods |
|---|
| Scanty positive | 12 | 10 (83.34%) (Including 2=BR) | 2=NINTP | 7 (58.33%) | 4=NG IN LJ1=CONT IN LJ | 06 (50%) | 06 (50%) |
| 1+ | 44 | 42 (95.45%) | 2=CONT | 40 (90.9%) | 4=NG IN LJ | 39 (88.63%) | 05 (11.36%) |
| 2+ | 52 | 50 (96.14%) (Including 1=BR) | 1=CONT1=NINTP | 48 (92.3%) | 1=NG IN LJ3=CONT IN LJ | 46 (88.46%) | 06 (11.53%) |
| 3+ | 45 | 45 (100%) (Including 1=BR) | NONE | 42 (93.33%) | 1=NTM2=CONT IN LJ | 42 (93.33%) | 03 (6.67%) |
| Total | 153 | 147 | 06 | 137 | 16 | 133 | 20 |
| (96.07%) | (3.92%) | (89.54%) | (10.45%) | (87%) | (13.07%) |
(NG IN LJ: No growth on LJ media; NINTP: Non-interpretable result in DMTT; IAPM: Indirect agar proportion method; NTM: Non-tuberculous mycobacteria; BR: Borderline resistant; DMTT: Direct MTT assay; CONT: Contamination)
Finally after ruling out no growth and contamination in both the methods, 133 results were available for comparison and on statistical analysis concordance was 91.4%, 97.6%, 95.1% respectively for 1+, 2+ and 3+ grades. For scanty positives it was 100% but the number was small. Overall concordance was 95.48% (127/133). Discordant results were observed in six samples, where two were found to be sensitive by DMTT but resistant by IAPM and another four samples were resistant by DMTT but sensitive by IAPM. The sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) for DMTT was 95.74%, 97.37%, 98.9%, 90.24%, respectively.
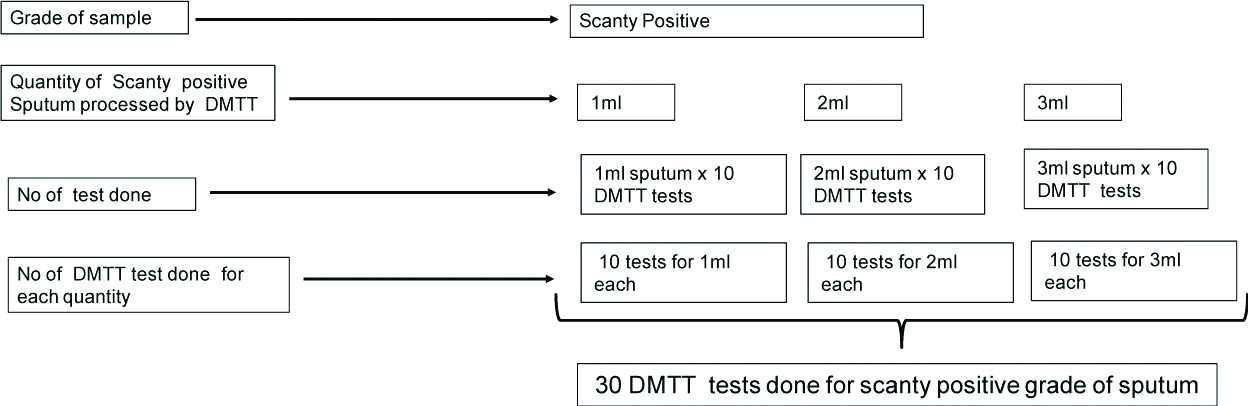
An interesting observation was made while analysing the results. The exact amount of sputum required for DMTT test has not been mentioned in literature. In this study, it was observed that out of 153 samples, more than 2 mL of sputum sample was available in 132 (86.27%) cases while in 21 (13.7%) the amount was 2 mL or less and yet on processing we could get results though delayed. Moreover, out of 3 non-interpretable results 2 were scanty positive and one was 2+ positive. Thus, a small exercise was undertaken to correlate the grading with the quantity of sputum in order to find out whether, in DMTT test, there was any relationship between them and the TAT. For every grade of sputum, 10 sputum samples were selected. Each sample in every grade was tested using 1 mL, 2 mL and 3 mL, quantity of sputum i.e., 30 tests were put up for each grade, making a total of 120 tests for all the four grades the methodology used was the same as for standardisation [Table/Fig-7].
Showing as example the algorithm followed for DMTT test done with respect to volume for scanty positive sputum samples (Total 30 samples).
The same protocol was followed for Grade 1+, 2+ and 3+, so total number of DMTT tests done for each grade=30 Tests. Total tests put up=120.
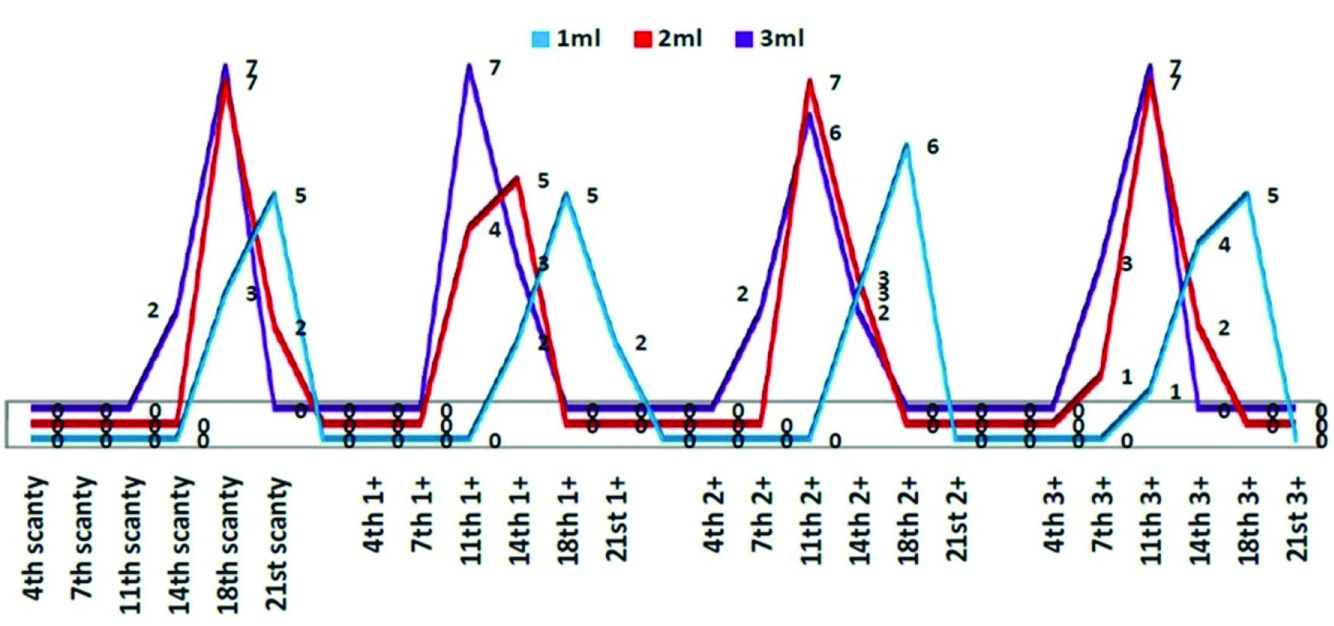
It was clearly observed that with scanty and 1+ positives sputum samples, when the quantity of the sputum was increased from 1 mL to 2 or 3 mL the TAT showed a shift from 21st to 14th day and the not interpretable results also decreased. Similar effect occurred in all grades of sputum [Table/Fig-8].
Showing DMTT test result with respect to grade of sputum, quantity and day of positivity.
Discussion
The developing countries have a high burden of MDR-TB [17]; therefore an economical, easy to perform test with minimum TAT requiring minimal equipment would be an ideal test for these countries. The researchers from Ethiopia have reported on the Direct MTT test [6,11,15] for the detection of drug resistance in M. tuberculosis and have also advocated that this test could be a reliable alternative method for rapid detection of drug resistant TB in high burden resource limited countries and could fill the gap between the availability and non-availability of the molecular methods [15]. The assessment of DMTT against grading of sputum positivity has not been reported before. Therefore, considering its utility for the peripheral centers, in the present study we have assessed the method against different grades of sputum, volume and TAT and reported that the test is quite sensitive; even with scanty and 1+ positive samples the interpretable results were 83.33% and 95.45% respectively. The overall interpretable results were 96% with only 1.96% each as not interpretable or contaminated.
Abate G et al., found 100% and WoldeMeskel D et al., 97.3% concordance for rifampicin susceptibility with their DMTT results when compared to IAPM [6,11]. A recent study also showed 99% concordance [15]. In the present study DMTT showed an overall 95.48% concordance with IAPM which was statistically significant (p<0.001). There were only 6/153 discordant results. Further the percent concordance was 92, 95, and 98 with 1+, 2+ and 3+ positive smears and the scanty positive samples gave 100% concordance, though the number for comparison was only six. The sensitivity of DMTT test was 95.74% and specificity was 97.37% with 98.9% PPV and 90.24% NPV which are good parameters for accepting the test for screening in light of the clinical findings. Another advantage of this method is that it can be interpreted by naked eye as our results corroborated 99% with resistant and 97% with sensitive strains. Therefore, in the absence of any instrument for taking the OD value the test can be safely read, making it a good test feasible even in poorly equipped laboratories.
The DMTT test in the present study and as shown by others helped to curtail with good concordance the TAT for reporting from 6 to 9 weeks in standard IAPM to 2-3 weeks [6,11,15]. In the present study 65.35% sample tested by DMTT gave interpretable results by 2nd week and by the end of 3rd week (21 days), all the interpretable samples could be read (n=147, 96.07%). This is a great advantage not only over the conventional standard IAPM, but also over the Indirect MTT method which also takes minimum of 3 to 7 weeks [13]. Abate G et al., have shown 63% interpretable results on the 7th day. They probably had samples with high bacillary load [6]. We had only one interpretable result on the 7th day and that was with three plus positive sputum.
It was interesting to note that with every grade of sputum positivity the interpretable results of DMTT were better than IAPM with overall 96% and 89.54% respectively [Table/Fig-6]. For scanty positive samples the figures were 83.35% and 58.33%, which means that in IAPM, being a two-step process, there is considerable primary loss due to either no growth or contamination of the medium. In contrast with DMTT the same scanty bacilli, if resistant, are able to thrive in the OADC supplemented 7H9 liquid medium giving thereby, better results.
In the present study there were 10/153 samples which failed to give conclusive results; 4 were borderline resistant of which one was an NTM and the other 3 were found to be resistant on IAPM; 3 (1.96%) were not interpretable and 3 (1.96%) were contaminants. Not interpretable results with Direct MTT test have also been reported by Abate G et al., to the tune of 4.05% and by WoldeMeskel D et al., at 3% [6,11]. Those samples that were reported as not interpretable by them had also not grown any bacteria when cultured on solid medium. In the present study also all the 3 sputum samples read as not interpretable did not produce any growth on LJ. Though, Abate G et al., and Wolde Meskel D et al., have not given the smear grading of these samples, we observed that 2 of the 3 belonged to the grade scanty positive and one was 2+ positive [6,11]. All the studies used Petroff’s method for concentration which is quite harsh on the bacteria [18]. It is quite possible that if softer N-acetyl- L-cysteine method is used instead, the interpretable results could improve.
Another important point brought out in the study is that the reading of results for scanty positive samples can be enhanced by increasing the quantity of the sample to about 5 mL, which can be done by refrigerating the first sample and collecting the second sample a few hours later or even the next day and then processing the two together. Increase in the volume of sputum is directly proportionate to the decrease in the TAT essentially due to more number of bacilli being available for growth and formation of formazan.
Limitation
Alone rifampicin resistance is considered as a surrogate marker for Multi-Drug-Resistant Tuberculosis (MDR-TB), but ideally MDR-TB is described as resistance to rifampicin and isoniazid and in this study we have not looked for isoniazid resistance which can be considered as a limitation. However, the methodology described to standardise the test based on sputum smear grading, can be used by researchers in evaluation of resistance to other drugs, particularly for those for which molecular tests are yet to be fully established.
Conclusion and Recommendation
We therefore conclude that after standardisation, the DMTT tube test on clinical samples with different grading of AFB positivity on sputum smear microscopy, including the scanty positive and one plus positive sputum samples, gave good concordance with the gold standard. With high sensitivity, specificity, positive and negative predictive values and along with the fact that it can be easily read by naked eye, the DMTT tube test for rifampicin can be recommended as a screening method for MDR-TB for use in the peripheral health setup where due to lack of skilled personnel and heavy power cuts, sophisticated laboratories for Cartridge Based Nucleic Acid Amplification Test (CBNAAT), Mycobacteria Growth Indicator Tube (MGIT) and Line Probe Assay (LPA) cannot be established.
Authors Contribution
PC, PN and UG- conceived, designed, executed and prepared the draft manuscript,
PC, PN, UG, RN and DKM- All critically evaluated the draft and finalised the version for publication.