Exostosis is defined as cartilage-capped lesion of bony outgrowth on the exterior of bone. As per World Health Organisation (WHO) definition, its medullary cavity remains in continuity of the parent bone [1]. Hereditary pattern of exostoses is called HME or diaphyseal aclasis and delineated by the presence of numerous exostoses [2]. Clinical and radiological features suffice for the diagnosis of HME and addendum is histopathology, if available. HME is an interdisciplinary complex pathology, so it necessitates that, orthopaedician, paediatrician and geneticist should work together for the diagnosis, research and treatment. While medical science is improving day by day but the management of genetic diseases like HME is still having several clandestine affairs. Trailing 25 years, the genetics and cellular biology have evolved much about HME, but still, the interactions among the cells and molecules at the growth plate are not elucidating [3].
The treatment of HME is grossly surgical. Surgery is needed only in symptomatic cases to avoid eventual intricacy. Limb length equalisation, deformity correction by osteotomy and epiphysiodesis are frequently entertained procedures in younger. Sometimes, multiple (up to 20) surgical intervention is needed for symptomatic exostosis resection [4]. There is scarcity of literature, mentioning the different clinical spectrum and management of HME at a single platform. So the present authors compiled the treatment and outcome of HME patients and analysis the data from an orthopaedic stance. The present study aimed to discuss the various presentation of secondary complication developed in HME patients and the treatment adjudication.
Materials and Methods
It was a prospective interventional study of 17 cases included in the study conducted between February 2012 to May 2018. In this study, all patients (irrespective of their the age, sex) of presenting complaints related to HME (i.e., bony swelling with decreased range of motion, sudden growth of previous swelling, difficulty in squatting and cross leg sitting etc.,) were included. Study excluded those patients who had come to hospital due to other complaints rather than HME related problems. The variables of the study were: age, sex, deformity, aesthetic issue, joint involvement, extremity involvement, secondary changes and treatment administered. The epidemiology of study design is depicted in [Table/Fig-1]. Classification system of HME is based on number of bone involved, skeletal deformity and with or without functional deficit [5].
Demographic profile of study population with complaints, clinical classification, treatment and SAPS score.
| S.no | Age/Sex | Presentation | Clinical classification type | Treatment executed | SAPS score |
|---|
| 1 | 21/M | difficulty in squatting | II A | excision of exostoses | S |
| 2 | 14/F | scapula alata | II B | excision of exostoses | S |
| 3 | 45/M | malignant changes in exostoses | II B | above knee amputation | S |
| 4 | 30/F | genu valgum and difficulty in squatting | III B | excision of offending exostoses | DS |
| 5 | 24/M | psychosocial issue | I B | excision of culprit exostoses | S |
| 6 | 17/M | difficulty in supination | IIIA | life style management | DS |
| 7 | 14/M | tingling of common peroneal nerve | I A | excision of offending exostoses | S |
| 8 | 28/M | pain and swelling around knee | II B | bursa and exostoses excision | S |
| 9 | 22/M | psychosocial issue | II A | excision of culprit exostoses | VS |
| 10 | 20/F | genu valgum and cosmetic issue | III B | corrective osteotomy and excision of exostoses | S |
| 11 | 33/F | unable to cross leg sitting and squat | III B | excision of exostoses | S |
| 12 | 38/M | psychosocial issue | III A | excision of offending exostoses | VS |
| 13 | 15/M | genu valgum and difficulty in squatting | III A | corrective osteotomy and excision of exostoses | S |
| 14 | 15/M | pain and swelling around knee | 1 B | bursa and exostoses excision | S |
| 15 | 29/F | tingling of common peroneal nerve | III B | excision of offending exostoses | S |
| 16 | 27/F | unable to cross leg sitting and squat | II B | excision of offending exostoses | DS |
| 17 | 41/M | malignant changes in exostoses | II B | below knee amputation | VS |
Firstly, the patients were briefed about genetic nature of pathology, possible progression, secondary complication, prognosis and limitations of orthopaedic surgeon to treat it. When they got satisfied that surgical intervention is not a cure and is only procurement to rectify their problem, then the surgery was administered.
The present institute is a referral centre, provides tertiary care and transacts a fair number of patients from distant areas. So, most of the HME patients would have been referred or visited us after moderate to severe functional deficit in their lifestyle or could have developed complication due to exostoses. Each patient had a different problem requiring different mode of treatment for the same disease. Moreover, the involved limb with joint was not near normal pre-operatively, so authors could not apply any scoring system for the whole group. However, the individual patient’s post-surgical outcome satisfaction was obtained by Short Assessment of Patient Satisfaction (SAPS) score [6].
Furthermore, due to small sample size statistical calculation could not be done. So the present authors considered this study as descriptive study in which the individual profile of patient of HME could be outlined in tabulated form.
Results
All the patients were managed by individualising the surgery. Limited surgical execution was done to only symptomatic exostosis. Since the variables of the study were separate so the analysis through comparing the average values was not feasible. Total 17 cases (average age 25.4 years) were included in the study with a male and female ratio of 11:6. The average follow-up duration of study was 14 months (8-18 months). At the end of last follow-up the SAPS score (Minimum 0 to maximum 28 points) of each individual patient was obtained and categorised as very dissatisfied (0-10), dissatisfied (11-18), satisfied (19-26), very satisfied (27-28). Three patients (17.6%) were very satisfied, 11 patients were satisfied (64.7%), 3 (17.64%) patients were dissatisfied [Table/Fig-1].
Discussion
The first literature of HME was written by John Hunter in his “Lectures on the principles of surgery” in 1786 where he mentioned that it can occur in any bone of the body [7]. Boyer A in his paper in 1814, first time discussed the family affected by HME [8]. Interestingly, HME is not only limited to human but also affects horse, cat, lion and lizard. Huge exostoses over the scapula has been mentioned in literature [9,10].
HME is a monogenetic disorder with family anamnesis is 65% in all HME cases [11]. Mutation in exostosin genes (EXT-1 and EXT-2) is responsible for HME, and it is inherited in the family as an autosomal dominant condition [12]. Due to dominant inheritance of HME, disease is either due to haploinsufficiency and Loss-of-Heterozygosity (LOH). Alteration in EXT genes occurs due to mutations, chain elongations or non-sense coding and eventually production of faulty EXT glycosyltransferases enzyme, which down-regulates the Heparin Sulfate (HS). Ultimately the lower concentration of HS ensues exostoses. In 44-70% of the HME cases, the responsible gene is EXT-1, while in the rest of the cases the accountability goes to EXT-2. Not uncommonly but, both genes may mutated in same patients also [13,14].
Alvarez C et al., in his study of 10 families, found that carriers of EXT-1 mutated gene were found to have big exostoses, more limb deformities with shorter height. So the genotype-phenotype correlation occurs in patients of EXT-1 gene mutations which leads to higher degree of anatomical load [15].
The exostosis grows at about angle of 60° and in upward direction (proximal) from the growth plate, so it’s not subjected to axial load [16]. As per the Wolff’s law the remodelling of bone is in response to loading. The bone remodels itself and becomes dense to overcome the loading effect. The inverse is same, the offloading decrease the bone mass. So it is expected that offloaded exostoses must also follow the reverse Wolff’s law and must have remodelled and are supposed to regress by osteoclastic resorption [17]. However, the enigma still prevails, since the exostoses do not disappear. Exostoses are covered by periosteum which covers the cartilaginous top. Cartilaginous top has an epiphyseal- like chondrocyte carrying cap and the periosteal layer yields fibroblast-like cell which express FGF9, FGFR3 and Collagen Type IIa [18]. Thus, the active regeneration of exostoses continues, probably it explains how exostosis exempt itself from resorption.
An exostosis can be surgically created, by inverting the ring of LaCorix at the span of 60°. However, it disappears finally by resorption [19]. Study by Trebicz-Geffen found that surgically created exostosis does not express FGFR3 and down-regulated Indian hedgehog, which explains the spontaneous regression of it [20].
Though the exostoses are located at metaphysis, but the place of origin is still questionable. Proliferative zone (resemble chondrocytes) of exostosis, stains for PCNA (proliferative marker of S phase). Which suggest that exostosis retain, primitive epiphyseal character or epiphyseal disc is probable place of origin [21]. On the contrary, the histopathology of earliest micro-exostosis, within the periosteum suggest that the groove of Ranvier as probable source of origin. So the exostoses originate in proximity to epiphysis but never from it [22]. Milgram JW, in 1983 showed that exostoses originates from aberrant cartilaginous epiphyseal growth plate tissue, and takes the sub-periosteal location and proliferates autonomously and detaches itself from edge of growth plate [23]. Consequently, further researches (mouse model) support that ablated Ext-1 in growth plate brings ectopic cartilage in vicinity of epiphysis but never from it. So, it seems that most probable answer to the origin of exostoses is growth plate [24].
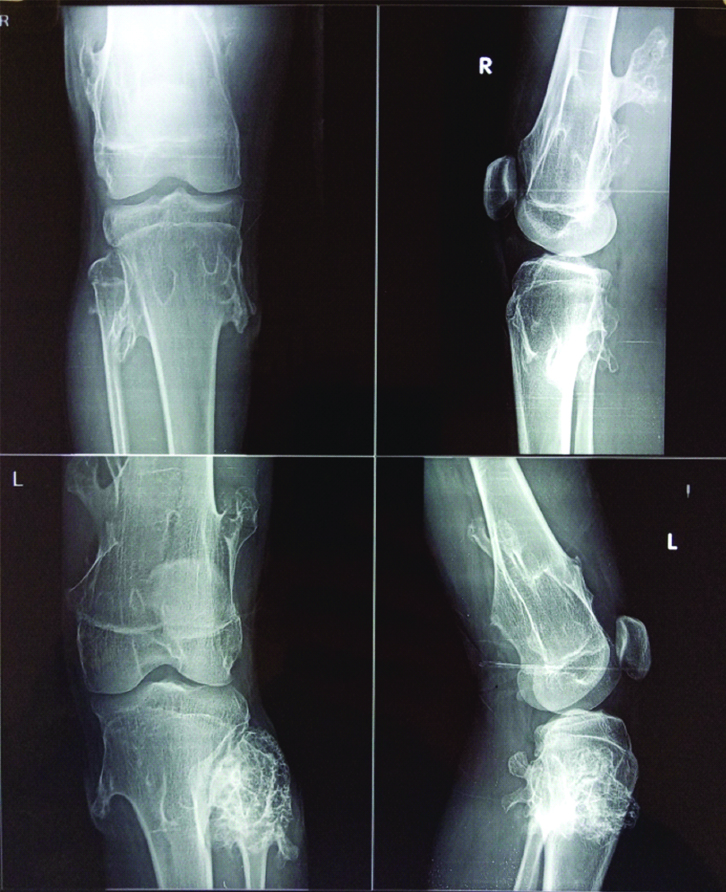
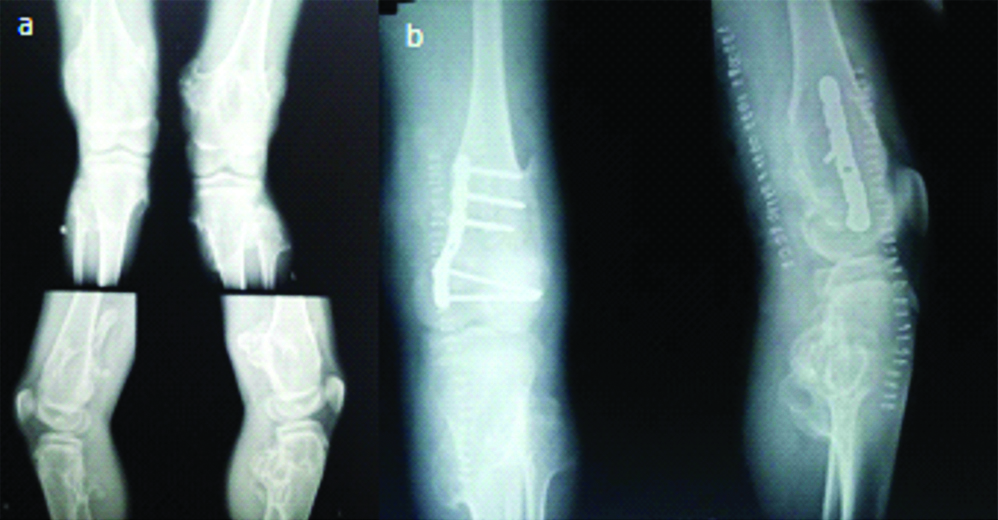
The affection of HME to general population is 1.5% [25]. Perhaps the incidence of HME is probably underestimated because the asymptomatic individuals are not involved in statistics. HME symmetrically afflicts and has male predilection (1.5-1), perhaps due to an easily overlooked milder female phenotype [26]. Clinical presentation of HME is varied in patients, though the few of them also remains asymptomatic. Usually, at the time of hospital visit, patient comes with the average number of six exostoses. Generally, the symptoms due to HME arise before the completion of sixth birthday in 89% of cases [27]. Since the knee joint and scapula is the most conspicuous site in children, so these are the first most common sites to be reported by parents [28]. In the present study, all the patients had a severely affected knee joint [Table/Fig-2]. More recently the HME has been classified into three types [Table/Fig-3].
Severe affection of knee joint by HME in 21-year-old male.
Clinical classification system for HME [4].
| I No deformities and no limitations | A ≤5 sites with osteochondromas functional |
| B >5 sites with osteochondromas |
| II Deformities and no functional limitations | A ≤5 sites with deformities |
| B >5 sites with deformities |
| III Deformities and functional limitations | A Functional limitation of 1 site |
| B Functional limitation of >1 site |
The first and very close differential diagnosis of HME is Type II Langer-Giedion syndrome (trichorhino-phalangeal Syndrome) followed by meta-chondromatosis and dysplasia epiphysealis hemimelica (Trevor disease). Trichorhino-Phalangeal Syndrome (TRPS) shares the typical characteristic features apart from several osteochondromas. It can be easily diagnosed due to thin nails, thick eyebrows, broad nose with rounded tip; thin upper lip and the affected one almost always have sparse scalp hairs [29]. Metachondromatosis is typified by multiple exostoses of hand and feet and enchondroma of long bone metaphysis and iliac crest [30]. Dysplasia epiphysealis hemimelica is a rare non-hereditary, developmental cartilaginous overgrowth (histopathologically indistinguishable from HME) from carpal and tarsal bone of single extremity in young children [31].
Invariably majority of HME patients seek hospital visit for pain, due to tendon or muscle compression, impingement, bursa and tendon rupture [32]. Pedunculated osteochondroma may be fractured due to muscle pull without history of trauma [33]. Often encountered repercussion of exostoses is restricted motion. In forearm, supination movement is mainly restricted due to exostoses in interosseous membrane, shortening of ulna, and more curved radius. Unbalanced shortening of ulna may cause dislocation of radial head and this deformity is called as Madelung-type deformity [34].
Maximum flexion of knee joint is hampered by exostoses in popliteal region. Almost one-third of HME cases develop genu valgum. Although the tibial metaphysis is most often responsible for it but the femoral component might also be present [35]. The exostoses tend to grow as well as the new one is formed, but the growth ceases after the closure of growth plate [36]. Among two variant of sessile and pedunculated form, the later one has more tendency to be problematic, due to more overlying tissue that it has [37].
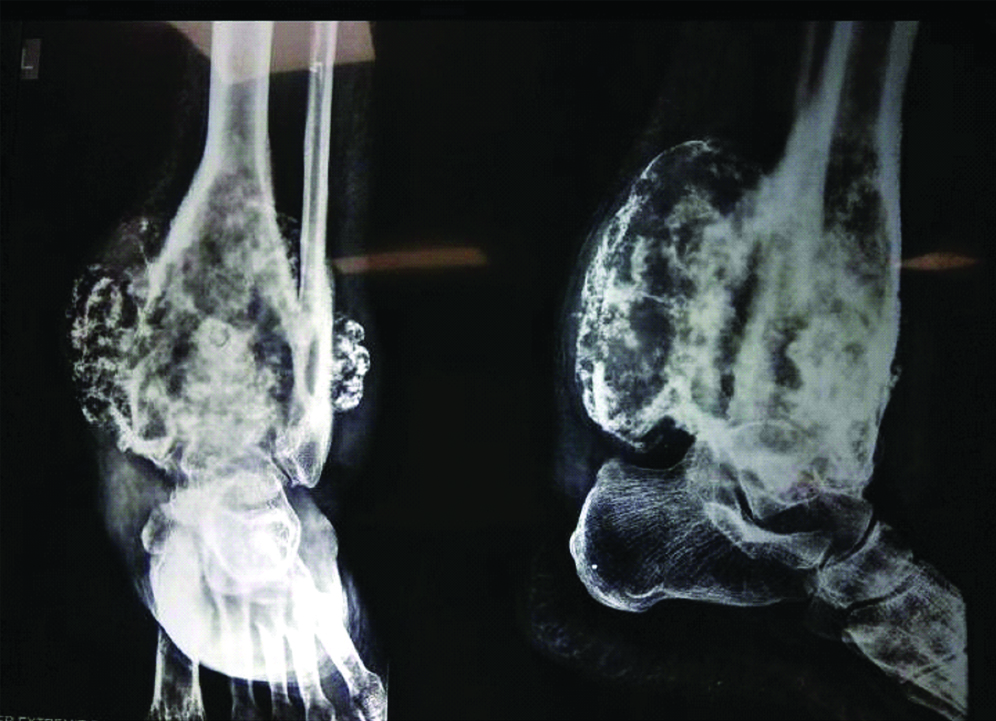
The usually encountered complication in day to day life due to HME is, hampered joint function. Other complications are malignant degeneration, typically restricted movement of elbow, bursa formation, misalignment of axial skeleton and local pressure effect. Average reliable estimate of malignant degeneration in HME patient is 0.5 to 2.0% [38]. In the Ochsner series, mean age of malignant degeneration was 31 years [39]. In the present series, two patients developed the malignant degeneration in long-standing osteochondroma [Table/Fig-4]. Malignant changes (chondrosarcoma) occurred after 35 years of existing osteochondroma in one patient and the patient got treated by amputation.
X-ray of patients showing malignant degenerative changes in long standing osteochondroma arising from distal tibia. Biopsy revealed the osteosarcoma and treatment executed in the form of amputation.
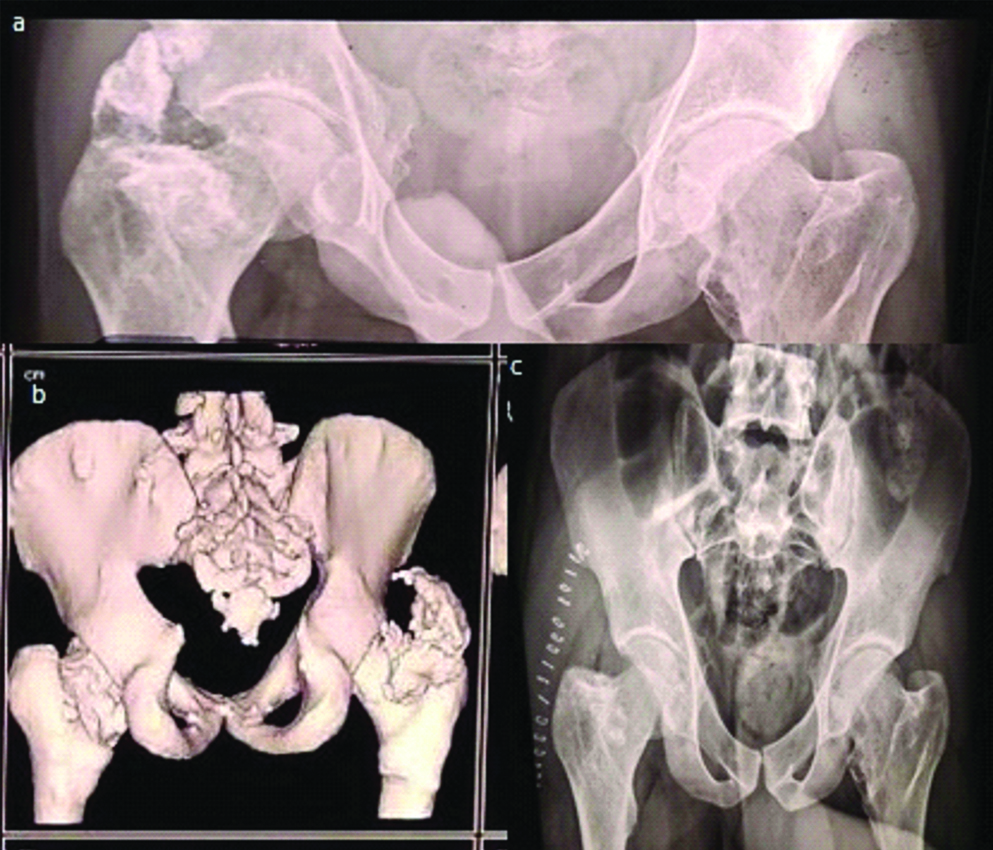
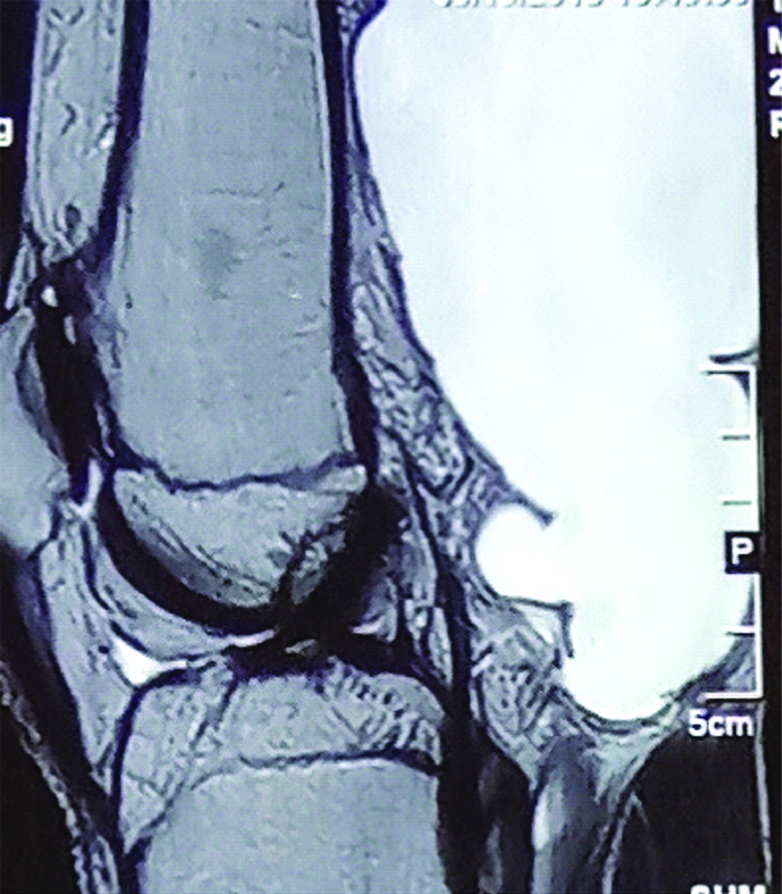
X-ray and CT-scan revealed the large osteochondroma as a culprit arising from neck and greater trochanter of femur which was impinging the acetabular roof. After resection, patient was able to discharge own ritual by squatting [Table/Fig-5]. Sometimes adventitious bursa develops in HME patients, which may rupture or get infected. In the present study two patients presented to us with infected bursa. Both of them were managed by excision of bursa and relevant osteochondroma [Table/Fig-6]. Two patients had chief complaints of difficulty in using Indian style lavatory and awkward gait. X-ray showed that distal femur component was responsible for genu valgum and multiple osteochondromas from posterior aspect were hindering the flexion of knee. By single incision, all offending part was removed and genu valgum was corrected by close wedge osteotomy [Table/Fig-7]. A 14 years old HME female patients presented to us with static type of winging of scapula. She only complain of an unaesthetic back. On investigation, she had ventral exostosis at medial border of scapula, responsible for winging of scapula and the offending exostosis removed finally [Table/Fig-8].
One of the HME patients had difficulty in squatting and cross leg sitting due to osteochondroma arising from (a,b) posterior part of right greater trochanter and part of base of neck of femur. Posterior approach used to excise the culprit lesion.
T2 weighted SAG MRI showing large bursa due to osteochondroma in HME.
Patient had (a) gross restriction of flexion movement at left knee and genu valgum. He got managed by (b) excision and corrective osteotomy.
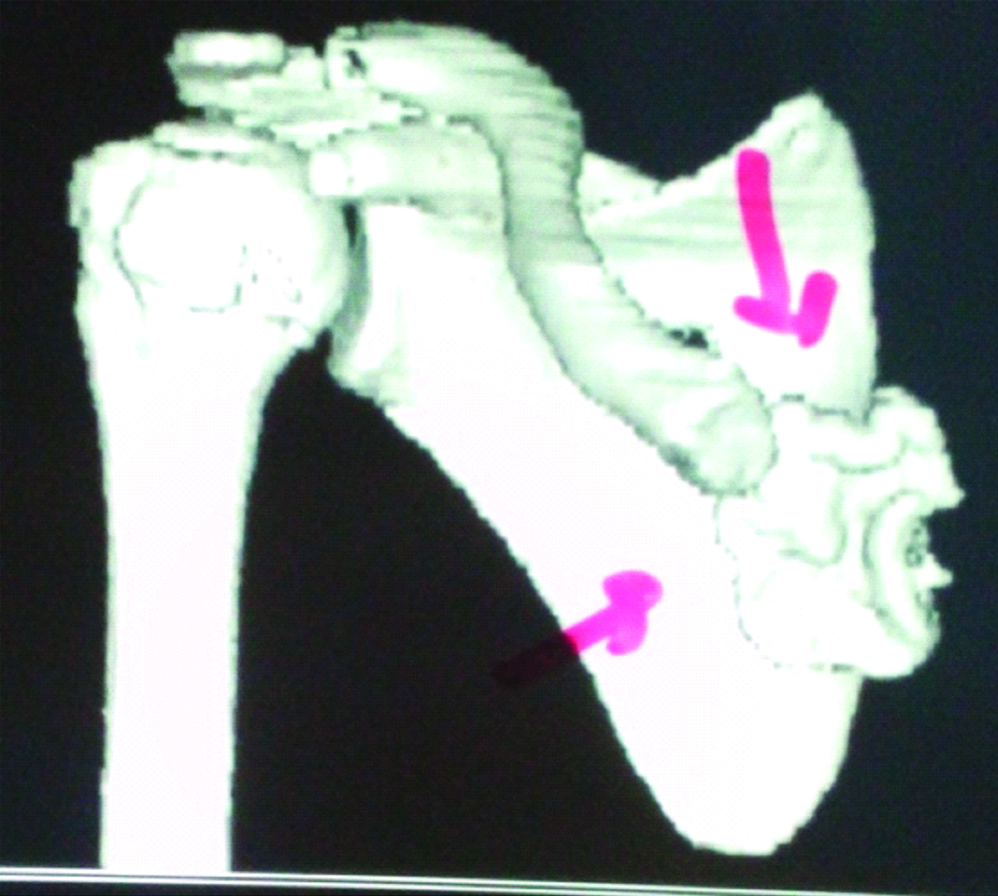
CT of a 14-year-old female having ventral exostosis at medial order of right scapula causing static winging of scapula.
Pelvic complications are not uncommon in HME patients. It sometimes has created difficulty in pregnancy. In the present cases, one patient of HME, presented to institute with difficulty in squatting and cross leg sitting.
Since HME is a genetic pathology, recent researches are trying to individuate useful therapeutic locus. Distinctively the triparanol (IHH blocking agent) is supposed to be used as neo-adjuvant therapy to down the tumour burden before surgery is under trial [40]. Heparanase is a protein easily traceable in growth plate (hypertrophic zone) of normal individual and promotes the chondrogenesis and BMP signaling. Huegel J et al., in his mouse model experiment emphasised that strong heparanase inhibitor, which potently inhibited chondrogenesis. So it seems that heparanase may be a conceivable therapeutic agent in near time to come [41].
Limitation
Due to limitate number of cases authors could not encounter the other mentioned secondary complications in HME cases like vascular compromise, Spontaneous haemothorax, and obstetric problems. Since these figures were collected at tertiary care centre, so there are higher chances that authors will be getting the only symptomatic patients or with secondary complications. So the present authors may be severely biased towards complication rate. So, the present study should not be conceded to take the inference for rate of complications in HME patients.
Conclusion
The orthopaedician is usually the first treating doctor who faces to diagnosis and treat the HME patients. So it necessitates that orthopaedist should be cognizant to clinical display of it, along with its differential and aftermath. Surgical mediation is often needed to excise the clinically obliged exostoses only. Seldom the deformity correction in weight-bearing bone and obstruction removal are necessitated by surgical intervention. Management of malignant transformation in HME is also a dictatorial regime of orthopaedic surgeon.
Fortunately, the ongoing research definitely in near future will allow us to better cognize the role of heparin sulfate in signal transduction as well as we will be concentrating for EXT-genes as tumour suppressor with important role in angiogenesis and malignant transformation.