Lower respiratory tract infections are a leading infectious cause of death worldwide, according the recently published 2015 Global Burden of Disease Study, with rates increasing in many developed countries [1]. CAP refers to infections acquired in the community, which excludes healthcare-associated disease. Mild cases can be treated successfully at home, but severe cases require hospital admission and are associated with greater cost and suffer higher mortality [2].
IgM ELISA is the most appropriate test but does not exclude immediate past infection and sub clinical illness. Thus, diagnosis of Mycoplasma pneumoniae infection is currently based principally on serology. The detection of IgM provides an early and sensitive diagnosis where as, PCR test is more reliable, but is expensive and is not easily available.
The aim of the study was to compare indirect Immunofluorescence Assay (IFA) with the ELISA for detection of IgM antibodies for the rapid diagnosis of Mycoplasma pneumoniae in a clinically suspected CAP cases.
Materials and Methods
A cross-sectional study was conducted from January 2016-January 2018 for a period of two years. All the suspected cases of CAP from inpatients and outpatients attending JSS Hospital, Mysuru were enrolled in the study. The inclusion criteria for this study were presence of at least one of the major clinical criteria as follows-cough with difficulty in breathing, sputum production, fever >37°C or two of the minor criteria chest pain, altered sensorium, signs of pulmonary consolidation, crackles on auscultation. The inclusion criteria are in accordance with Infectious Disease Society of America/American Thoracic Society (IDSA) guidelines on the management of CAP [2].
Presence of pulmonary infiltrate/shadow on chest X-ray is suggestive of pneumonia within 24 hours of hospitalisation, and patients residing in the community with similar symptoms where included in the study.
Exclusion criteria for this study were patients who had received macrolides as antimycoplasma therapy and pneumonia developed 72 hour after hospitalisation.
The ethical permission was obtained from JSS Medical College with registration number JSSMC/IEC/07/4496/2016-17.
A written consent was taken from the adults and for the paediatric population consent was taken from the parent or a guardian before enrolling into the study. A detailed history and clinical examination was performed in all the clinically suspected cases of CAP, radiological findings were done for all the cases and the details were entered to the pro forma. A single blood sample was taken from the patients during the time of enrolment to the study.
Blood samples of 1-2 mL was collected from the patients for the detection of IgM antibodies against Mycoplasma pneumoniae. Serum was separated and stored at -20°C till the sample was processed.
Serology
ELISA was carried out for the serum IgM antibodies against Mycoplasma pneumoniae using commercially available ELISA kits. The procedure was followed as per the manufacturer’s instructions (Vircell microbiologists). The interpretative criteria were consistent with the recommendations of the manufacturer as outlined on the package insert. IFA for immunoglobulin M, the principle of the test is based upon antibodies present in the sample and its interaction with the antigen coated on the slide surface. The binded antigen-antibody complexes react with the fluorescein labelled anti human globulin and the slides are examined using an immunofluorescence microscope. The procedure was followed as per the manufacturer’s instructions (Vircell microbiologists,).
Procedure
Patient’s sample (25 μL) was diluted with (25 μL) of Phosphate Buffered Saline (PBS), Diluted sample was treated with anti-human IgM sorbent by adding 5 μL of diluted patients serum+25 μL of sorbent mixed thoroughly. A 20 μL of sorbent treated sample was then added to every slide well. The slides were placed in the humid chamber and incubated at 37°C for 90 minutes.
The slides were rinsed with PBS and immersed for ten minutes in the PBS. Then the slides were air-dried and 20 μL of anti-human IgM Fluorescein Isothiocyanate (FITC) conjugate solution was added to each well. The slides were then incubated in humid chamber for 30 minutes at 37°C. Slides were rinsed with PBS and immersed for 10 minutes in the PBS, later dipped and washed with distilled water. Small drop of mounting medium was added and covered the slides with cover slip. The slides were read in a fluorescence microscope at 400x magnification. The [Table/Fig-1] showing the positive IFA Mycoplasma pneumoniae preparation.
Test samples showing positive apple green fluorescent aggregates of bacteria on the background of red stained cells.
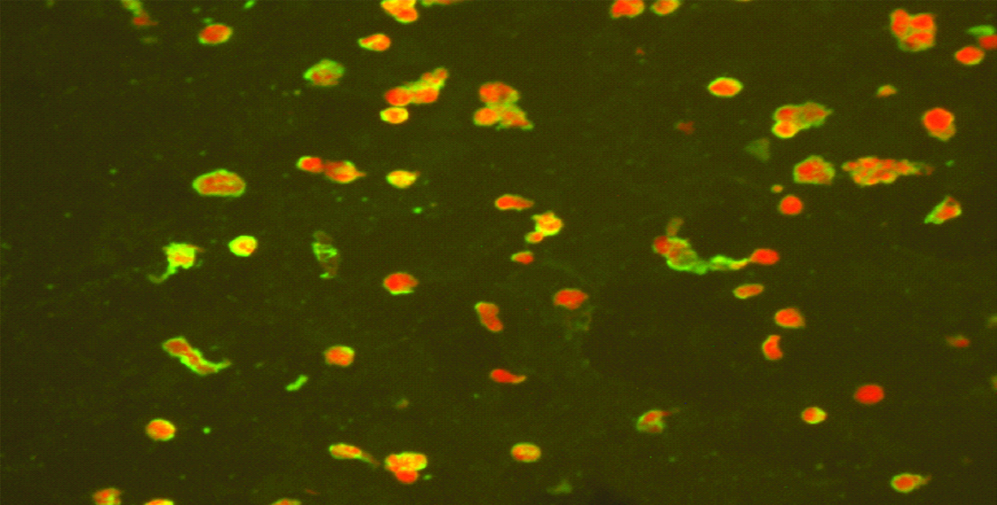
Results
A total of 200 patients were enrolled in this study out of which 108 (54%) were males and 92 (46%) were females. Among which 123 (61.5%) constituted of paediatric population and 77 (38.5%) were adults respectively. Mycoplasma pneumoniae ELISA for immunoglobulin M was performed on 200 samples.
A total of 60 samples were positive for IgM antimycoplasma antibody. Out of 60 samples 11 (5.5%) samples were positive by ELISA and 60 (30%) samples were positive by immunofluorescence [Table/Fig-2]. With the ELISA as a gold standard sensitivity and specificity was found to be 100% and 74.1% of the tests. Positive predictive and negative predictive values are as follows 18.3% and 100%. The agreement between ELISA and IFA was low [Table/Fig-3].
Results of Mycoplasma pneumoniae Pneumonia IgM Enzyme linked immunosorbent assay and indirect immunofluorescence assay.
| Test | Positive | Negative |
|---|
| ELISA | 11 (5.5%) | 189 (94.5%) |
| IFA | 60 (30%) | 140 (70%) |
| ELISA+IFA | 11 (5.5%) | 189 (94.5%) |
Showing the sensitivity and specificity of IFA and ELISA.
| ELISA | Total |
|---|
| Positive | Negative |
|---|
| IFA | Positive | 11 (100%) | 49 (25.9%) | 60 (30%) |
| Negative | 0 (0.00%) | 140 (74.1%) | 140 (70%) |
| Total | 11 (100%) | 189 (100%) | 200 (100%) |
*Sensitivity 100% and specificity 74.1%
Most of the positive samples were from the age group of 0-10 years (71.6%) followed by 11-20 years (13.3%) [Table/Fig-4].
Association of Mycoplasma pneumoniae infection with age and sex in 60 patients with community-acquired lower respiratory tract infections.
| Age (year) | Male | Female | Total | Percentage (%) |
|---|
| 0-10 | 29 | 14 | 43 | 71.6% |
| 11-20 | 6 | 2 | 8 | 13.3% |
| 21-30 | 1 | 0 | 1 | 1.66% |
| 31-40 | 0 | 2 | 2 | 3.33% |
| 41-50 | 1 | 2 | 3 | 5.0% |
| 51-60 | 0 | 0 | 0 | 0 |
| 61-70 | 0 | 1 | 1 | 1.66% |
| 71-80 | 0 | 2 | 2 | 3.33% |
| >80 | 0 | 0 | 0 | 0 |
| 37 | 23 | 60 | 100% |
Clinical Profile of the Positive Samples
Cough (96.6%) was the most common symptom among the subjects positive for Mycoplasma pneumoniae followed by fever (85%), cold (41.6%), diarrhoea and abdominal pain (11.6%) [Table/Fig-5].
Crackles (56.6%), rhonchi (55%), murmurs (55%) were the most common clinical signs among the patients positive for Mycoplasma pneumoniae followed by creptations (51.6) [Table/Fig-6].
Showing clinical symptoms among Mycoplasma pneumoniae positive subjects.
| Clinical symptoms | Present (n) | Absent (n) |
|---|
| Fever | 51 (85%) | 9 (15%) |
| Cold | 25 (41.6%) | 35 (58.3%) |
| Cough | 58 (96.6%) | 2 (3.3%) |
| Head ache | 3 (5%) | 57 (95%) |
| Vomiting | 4 (6.6%) | 56 (93.3%) |
| Diarrhoea | 7 (11.6%) | 53 (88.3%) |
| Abdominal pain | 7 (11.6%) | 53 (88.3%) |
Clinical signs among Mycoplasma pneumoniae positive subjects.
| Clinical signs | Present | Absent |
|---|
| Wheeze | 28 (46.6%) | 32 (53.3%) |
| Coryza | 26 (43.3%) | 34 (56.6%) |
| Hurried breathing | 28 (46.6%) | 32 (53.3%) |
| Murmurs | 33 (55%) | 27 (45.0%) |
| Crackles | 34 (56.6%) | 26 (43.3%) |
| Creptations | 31 (51.6%) | 29 (48.3%) |
| Rhonchi | 33 (55%) | 27 (45%) |
Discussion
Mycoplasma pneumoniae is a leading agent of human respiratory infections. Symptoms range from mild and often undetected forms of tracheobronchitis to interstitial pneumonia. Mycoplasma pneumoniae is a common respiratory pathogen that produces diseases of varied severity ranging from mild upper respiratory tract infection to severe atypical pneumonia [8].
It is important to diagnose Mycoplasma pneumoniae in CAP patients by rapid tests which will help in initiating early treatment to prevent further complications. Empirical treatment is usually started as per the guidelines of CAP [2]. It is difficult to cultivate to Mycoplasma pneumonia and it is also time consuming. Hence the diagnosis is dependent either on serological tests or Immunofluorescence assay. However, serology is not reliable in specificity, needs paired sera, and is usually positive at about seven days after the onset of disease [9,10]. In contrast, Immunofluorescence assay may provide an early diagnosis of Mycoplasma pneumonia infection and could be used as a useful diagnostic technology.
In this study, we have focused on determining the utility of rapid tests for the detection of Mycoplasma pneumoniae and for appropriate use of antibiotics. Since, there are only few literatures on the prevalence of Mycoplasma pneumoniae pneumonia in south India this is the first of its kind study in this region of Karnataka as per best of the our knowledge. Hence, an attempt was made to know the rate of Mycoplasma pneumoniae pneumonia in this set up.
Overall, Mycoplasma pneumoniae was diagnosed in 60 (30%) patients out of 200 by ELISA and IFA. The present study was in consonance with the study conducted by Mathai E et al., and Ramamoorthi U et al., in which they had positivity of 30.4% and 31.5% respectively [11,12]. Among the 60 serology IgM positive samples by ELISA +IFA, 11 (5.5%) samples were positive by ELISA and 60 (30%) samples were positive by IFA. The agreement between the ELISA and IFA assays was low. Variables associated with discordant results between ELISA and IFA assays are as follows. Positive predictive value and negative predictive value 18.3%, and 100%, respectively. Similar results were found in the study by Gao S et al., where they found low agreement between ELISA (15.6%) and IFA (10%) [13]. They found ELISA to be better than IFA which is in contrast with our study where we found IFA to be efficient in giving positive results than ELISA in a clinically suspected cases.
In the present scenario there is no accepted gold standard serological test for the detection of antibodies against Mycoplasma pneumoniae. The present study elucidates the need to carry out various serological tests like Enzyme Immunoassay (EIA), IFA for the better understanding of sensitivity and specificity of the tests for the detection of Mycoplasma pneumoniae.
In the present study, among the 60 patients positive for IgM antibodies against Mycoplasma pneumonia we found the sensitivity, specificity, positive, and negative predictive values of ELISA for Mycoplasma pneumoniae were 100%, 74.1%, 18.3%, and 100%, respectively. The agreement between ELISA and IFA was low.
According to present study vircell ELISA had less sensitivity and specificity when compared with vircell IFA which is in consonance with the study Kim MH et al., where they found ZEUS Mycoplasma IgM ELISA had better diagnostic positivity than Vircell IgM Mycoplasma pneumoniae ELISA in the detection of IgM antibodies [14].
The present study also demonstrated that age had a major influence on the fraction of positive sera. The highest seropostivity for IgM antibodies against Mycoplasma pneumoniae was in the age group of 1-10 years which was concordant with the study Srifuengfung S et al., where most of the IgM positive cases were in the age group of 5-9 year (40.26%) [15], similarly with Shenoy VD et al., were most of the positive cases were in the age group of 5-10years [16].
The high IgM positivity in the group aged 1-10 years suggested strongly that single-IgM measurement may be helpful in efforts towards early diagnosis of Mycoplasma pneumonia infection. The percentage of Mycoplasma pneumoniae-positive patients were higher in 34 (32.3%) males than in 23 (28.5%) females and the incidence of Mycoplasma pneumoniae infection with sex was found to be statistically significant. The present study is in contrast with Kumar S, where the incidence of Mycoplasma pneumoniae was predominant in female (156) than male (135) [17].
On analysing the clinical signs of the patients, 96.6% of Mycoplasma positive cases had complained of cough followed by fever 85% which is similar to the studies conducted by Lii Zhou L et al., where they found cough (91.9%) and fever (71.5%) were the most common among patients positive for Mycoplasma pneumoniae followed by cold (41.6%) [18]. Similarly, even Zhang Y, in their study found cough (100%) and fever (86.3%) to be the most common symptom [19].
Clinical symptoms crackles, crepitation and rhonchi (56.6%), (51.6%) and (55%) were the most common among the patients positive for IgM Mycoplasma pneumonia which is in concordance with the study by Kumar S, where crepitation (31.4%) and rhonchi (23.3%) were the most common symptoms in the positive cases [17].
Limitation
Paired serum samples should be used for the detection of true recent infections. Combination of tests must be carried out along with significant suggestive clinical signs in the clinical suspected cases for the CAP. No single test can confirm the diagnosis of Mycoplasma pneumonie relying on the serological tests might lead to false positives. Thus further studies should be carried on the molecular detection of Mycoplasma pneumoniae for the better diagnosis.
Conclusion
The rate of infection of Mycoplasma pneumoniae was 30% in the present study. Thus, it is important to have routine diagnostic tests for the IgM antibodies against Mycoplasma pneumoniae in the clinically suspected cases for the better diagnosis and for the empirical treatment. There are only few data suggesting of Mycoplasma pneumoniae pneumonia infection in this region thus testing for atypical pathogens in the respiratory infection should also be encouraged. Since IgM antibodies takes approximately 7-10 days’ time to develop and also IgM is not significant in adults, since they develop weak IgM responses in the coarse of primary or reinfection, thus molecular methods also should be used for the early diagnosis of the infection.
*Sensitivity 100% and specificity 74.1%