Ethanol causes poisoning and death in cases of high consumption and is considered as one of the most important causes of mortality all over the world [1]. Extreme alcohol consumption is one of the most common causes in the development of hepatic cirrhosis [2]. Alcohol can cause malfunction of oesophageal, acute and chronic gastritis, change in liver fat and hepatitis, pancreatitis, suppression of immune system and functional disorders of the reproductive system [3]. Ethanol is substantially metabolised in the liver and several enzymes in the liver perform this function. The first stage of ethanol metabolism is its conversion to acetaldehyde [4]. In the liver, ethanol is metabolised primarily to Alcohol Dehydrogenase (ADH) isoenzyme and the microsomal ethanol oxidation system and the other ethanol metabolisation enzymes such as catalase. These secondary metabolites can cause damage to liver tissue and lead to the increase of some hepatic enzymes (ALP, ALT and AST) in the plasma [5]. Many studies have been carried out in the last decade on the medicinal plants and the production of drugs containing natural active ingredient has created new horizons to the society of the researcher physicians and pharmacists [6]. Sickleweed (Falcaria vulgaris) belongs to Apiaceae (Umbelliferae) family, grows wildly in the west and southwest of Iran and it is characterised by rapid growth and annual plants [7]. The photochemical studies of this plant have documented the presence of tannins and saponins. Also, it is evidenced to contain vitamin C, phytosterols, protein and starchy materials and it is applied for the curing of gastric ulcer [8]. The results of the studies by Holt RI, demonstrated that the antioxidant compounds in the F. vulgaris extract [9]. It seems that the main elements acquired from leaves and stems of the plant have shown antioxidant properties and neutralising activities of free radicals [10]. Oxidative stress seems to play a major role in liver toxicity damage. The antioxidants can be found in the stems and leaves of some herbs can probably protect the liver against oxidative lesions resulted by free oxygen radicals [11], based on the antioxidant properties of F. vulgaris and the availability and affordability of this plant as a vegetable in some parts of the world. Also, considering drinking alcohol in many societies and the toxic effects of ethanol and so far no research has been conducted on the evaluation of F. vulgaris effects on ethanol-induced lesions of the liver. So, the current research was designed to investigate the effect of F. vulgaris against toxic effects of ethanol on the liver of rats.
Materials and Methods
Animals: This experimental study was done from December 2017 to April 2018 in the Department of Anatomical medical school in Kermanshah University of Medical Sciences in Iran. Animal study was conducted according to the guidelines for the care and handling of animals prepared by the Iranian Ministry of Health. All investigations conformed to the Ethical and Humane Principles of Research and were approved by the Ethics Committee of Kermanshah University of Medical Sciences (ethics certificate No.1396.612). Sixty four male Wistar rats (220-250 g) were purchased from Pastor Institute of Iran. The animals were housed in standard cages (three per cage) and control conditions at 23±2°C and exposed to 12-hours light/dark cycle, in Medical Sciences University’s animal care facilities for a period of one week before testing and exposing to environmental and climatic conditions. The animals had free access to water and food during this period [12].
Preparation of F. vulgaris extract: The F. vulgaris medicinal plant was purchased from a local store and their impurities were removed. After confirmation by the botanist, the plants were cleaned and the leaves and stems were dried under shade for five days and then they were milled to acquire plant powders. Three hundred gram of powdered plant was mixed with Ethanol 90% (1:5 ratio). The solution was kept in dark for 72 hours in a hot water bath at 36°C. In the next step, the solution was gradually poured onto the filter paper of Buchner funnel and filtered through the vacuum pump device. This solution was transferred to a rotary device to remove its extraneous solvent for more concentration. The isolation process continued until a very concentrated extract was obtained. The extract from the process described above was dissolved in distilled water and administered into the animal through oral gavage at certain dosages [10].
Experimental design and treatments: Sixty-four male rats were randomly divided into eight groups and eight rats were placed in each group. First group: the normal control group that received normal saline (through oral gavage) equivalent to the amount of experimental groups. Second group: ethanol control group, each animal received normal saline with 20% ethanol single dose, through gavage once a day for 28 consecutive days [13]. Third to fifth groups: the F. vulgaris groups, in these groups, each animal respectively received (50, 100 and 150 mg/kg) of F. vulgaris through gavage, for 28 days at 10 am [10]. Sixth to eighth groups, ethanol+F. vulgaris groups, in these groups, each animal received single dose of ethanol in order to induce liver damage, then they respectively received (50, 100 and 150 mg/kg) of F. vulgaris through gavage for 28 days, at 10 am.
Collection of blood serum and liver weight measurement: Animals of each group were placed one after another in a plastic container in a packet of cotton covered with Ether for 24 hours. They were anaesthetised due to inhalation of ether fume. Blood samples were taken by cardiac puncture from right ventricle. The samples were incubated at 37°C to clot. The coagulated samples were then centrifuged at 5000 rpm for 15 minutes to separate the serum. The parted serum was kept at -18°C for measurement of the Nitric oxide and biological liver factors levels. Animals were killed and sacrificed. Livers were removed and weighed on a microbalance sensitive to 0.001 mg (Switzerland) and middling weights of the livers of rats were recorded [14].
Biological Analysis
The liver was split and turned into a uniform solution. To separate the biological enzymes, the obtained solution was centrifuged at 10,000 rpm for 15 minutes twice. The supernatant was separated to measure the enzymes. ALT and AST actions were examined by the method of Reitman and Frankel. ALP actions were determined according to the procedure set out in the practical laboratory manual [14].
Serum Nitric Oxide (NO) assay: Nitric oxide, measurement was done by Griess assay using microplate technique [6]. To measure concentration of nitric oxide in the serum, after de-freezing the serum samples, in this examination, supernatant (500 μL) was deproteinised by zinc sulfate (7 mg zinc sulphate powder was mixed with 500 μL serum and vortexed for 2 minutes) with centrifugation. A 200 μL supernatant was taken and 200 μL vanadium chloride, 60 μL N-(1-naphthyl) Ethylenediamine Dihydrochloride (NEED) and 60 μL sulfonamide solutions were added. Sodium nitric (0.2 M) was used for the standard curve, and increasing concentrations of sodium nitric (5, 10, 25, 50, 75, and 100 μM) were prepared. The Greiss solution was added to all microplates containing sodium nitric and supernatant and was read through an ELISA reader (stat fax100. USA) at the wavelength of 540 nm [7].
Histological examinations and morphometric measurements: The inferior 1-cm-long part of the right lobe of the liver in transverse pieces was removed, washed in saline, and fixed in 10% formalin, dehydrated in ascending concentration of ethanol, cleared in xylene, and then embedded by paraffin. Thin sections (4 mm) were cut using a microtome (Leica RM 2125, Germany) and marked with haematoxylin and eosin. For each hepatocyte, the full cellular area was measured. The hepatocyte outline was measured after capturing an image with a ×40 objective. The maximum and minimum axis was measured in the drawing of each hepatocyte for measuring the mean axis. At least 50 hepatocytes from each zone were measured in each liver. A separate measurement for Central Hepatic Vein (CHV) was performed using the same assay. The planning was examined with an Olympus BX-51T-32E01 research microscope connected to a DP12 Camera with 3.34-million pixel resolution and Olysia Bio software (Olympus Optical Co., LTD., Tokyo, Japan) [14].
Fluorescence Recovery After Photobleaching (FRAP) method: FRAP method was used to measure the total antioxidant capacity of the serum. In this technique, the ability of the plasma to reinstate ferric ions was measured. This process required a great quantity of FeIII. A blue stain was formed when the compound of FeIII-TPTZ in acidic pH returned to FeII and absorption at the maximum wavelength of 600 nm. The factor defining the speed of the FeII-TPTZ and blue colour was only the vitalising power of the sample. Total antioxidant capacity values are strategised by means of the standard curve with diverse concentrations of iron sulfate [15].
Statistical Analysis
After extracting the information, Kolmogorov–Smirnov test was first conducted to confirm the data compliance of the normal distribution. One-way analysis of variance (one-way ANOVA) was used for statistical analysis and Tukey post-hoc test was used to determine the difference between the groups. Statistical Package for the Social Sciences (SPSS) 16.0 was used for data analysis, and the results were expressed as mean±standard error, and p<0.05 was considered significant.
Results
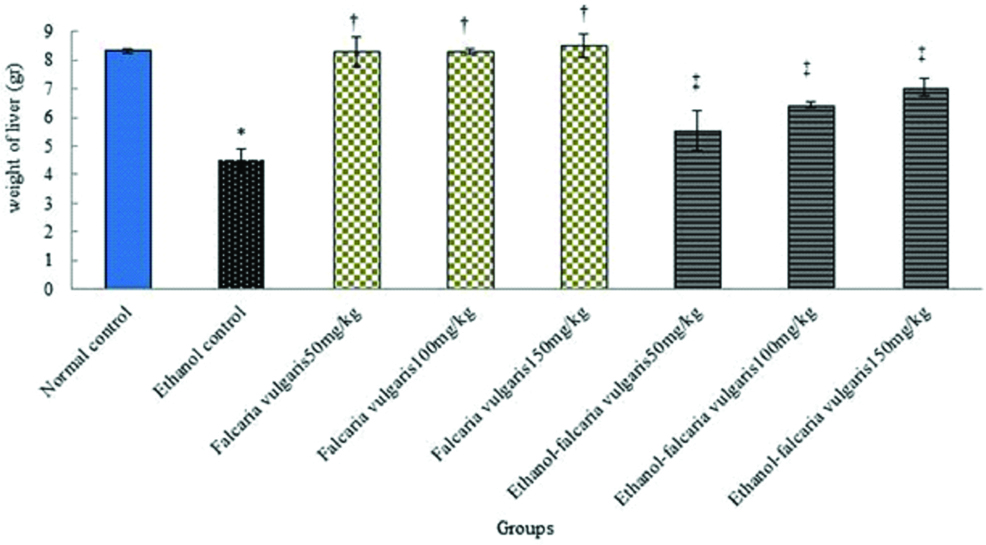
Weight: Ethanol led to a significant decrease in liver weight in ethanol control group compared to the normal control group (p<0.05). The mean of liver weight was not significant in all F. vulgaris groups compared to the normal control group (p>0.05). The mean of liver weight increased significantly in animals treated with F. vulgaris and F. vulgaris+ethanol administration at all doses in comparison to the ethanol control group (p<0.05) [Table/Fig-1].
Effect of ethanol, F. vulgaris and F. vulgaris+ethanol administration on weight of liver. *Significant decrease of weight for ethanol control group compared to the normal control group (p<0.05). †Significant increase for all F. vulgaris groups compared to the ethanol control group (p<0.05). ‡Significant increase for all F. vulgaris+ethanol groups compared to the ethanol control group (p<0.05).
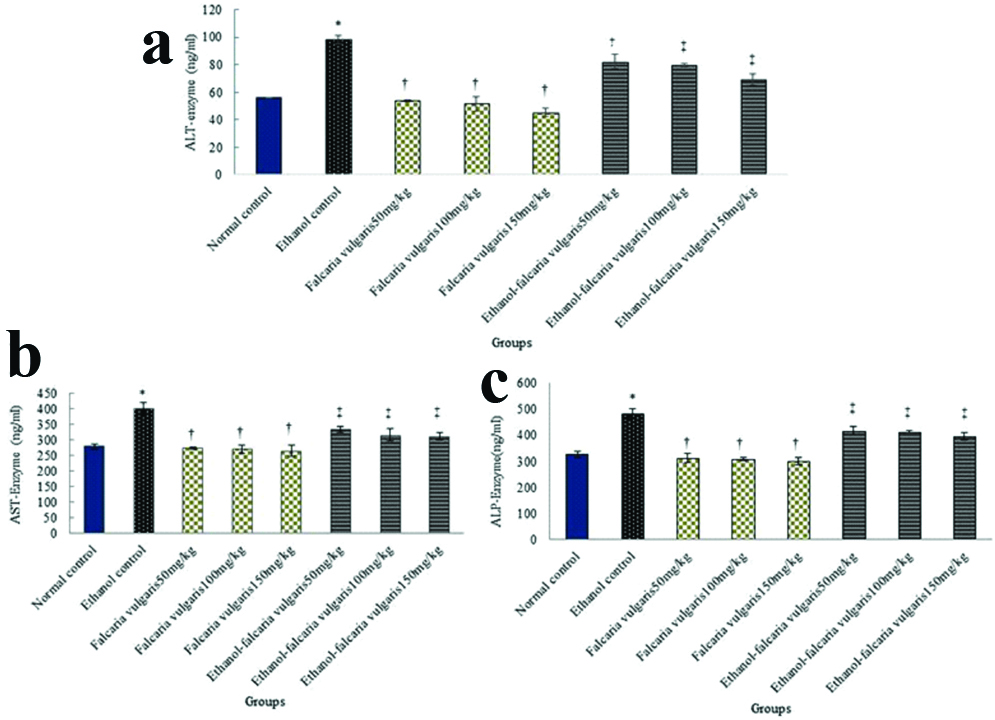
Biological factors: Ethanol led to a significant increase in ALT, AST and ALP enzymes in ethanol control group compared with the control normal group (p<0.05). The mean concentration of ALT, AST and ALP enzymes was not significant in all F. vulgaris groups compared to the normal control group (p>0.05). Also, the mean of ALT, AST and ALP enzymes in all F. vulgaris and F. vulgaris+ethanol groups decreased significantly compared to the control ethanol group (p<0.05) [Table/Fig-2].
Effect of ethanol, F. vulgaris and F. vulgaris+ethanol administration on liver enzymes. a) ALT enzyme; b) AST enzyme; and c) ALP enzyme. *Significant increase of enzymes for ethanol control group compared to the normal control group (p<0.05). †Significant decrease for all F. vulgaris groups compared to the ethanol control group (p<0.05). ‡Significant decrease in all F. vulgaris+ethanol groups compared to the ethanol control group (p<0.05).
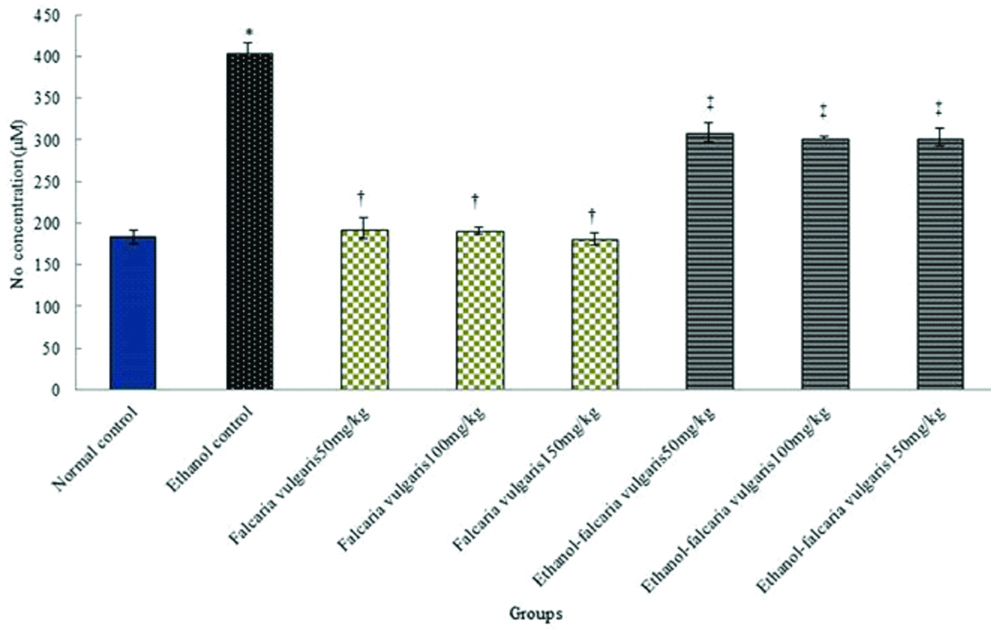
Nitric oxide: The results of blood serum nitric oxide measurement showed a significant increase in control ethanol group compared to the control normal group (p<0.05). The mean nitric oxide in the blood serum was not significant in all F. vulgaris groups compared to the normal control group (p>0.05). Also, the mean of nitric oxide in blood serum reduced significantly in F. vulgaris and F. vulgaris+ethanol administration at all doses compared to the control ethanol group (p<0.05) [Table/Fig-3].
Effects of F. vulgaris, ethanol and F. vulgaris+ethanol on the mean nitric oxide levels. *Significant increase of nitric oxide for ethanol control group compared to the normal control group (p<0.05). †Significant decrease for all F. vulgaris groups compared to the ethanol control group (p<0.05). ‡Significant decrease for all F. vulgaris+ethanol groups compared to the ethanol control group (p<0.05).
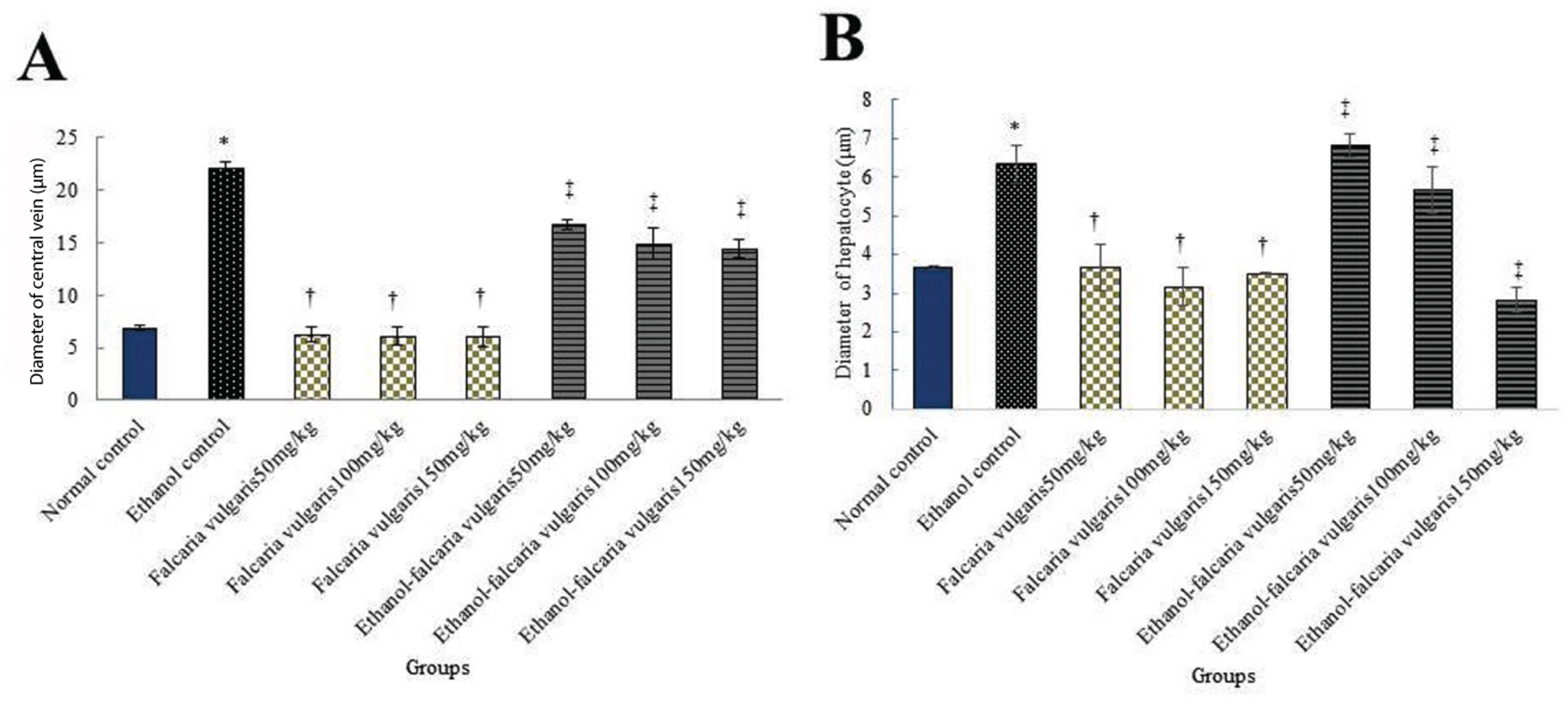
Morphometric: The mean diameter of hepatocytes and CHV showed a significant increase in ethanol control group compared to the normal control group (p<0.05). The mean diameter of hepatocytes and CHV was not significant in F. vulgaris groups compared to the normal control group (p>0.05). Further, the mean diameter of hepatocytes and CHV in all F. vulgaris and F. vulgaris+ethanol groups reduced significantly compared to the ethanol control group (p<0.05) [Table/Fig-4].
Effect of ethanol, F. vulgaris and F. vulgaris+ethanol administration on the mean central hepatic vein (a) and hepatocytes (b) diameters. *Significant increase of the mean hepatocytes and central hepatic vein diameters in ethanol control group compared to the normal control group (p<0.05). †Significant decrease for all F. vulgaris groups compared to the ethanol control groups (p<0.05). ‡Significant decrease for all F. vulgaris+ethanol groups compared to the ethanol control group (p<0.05).
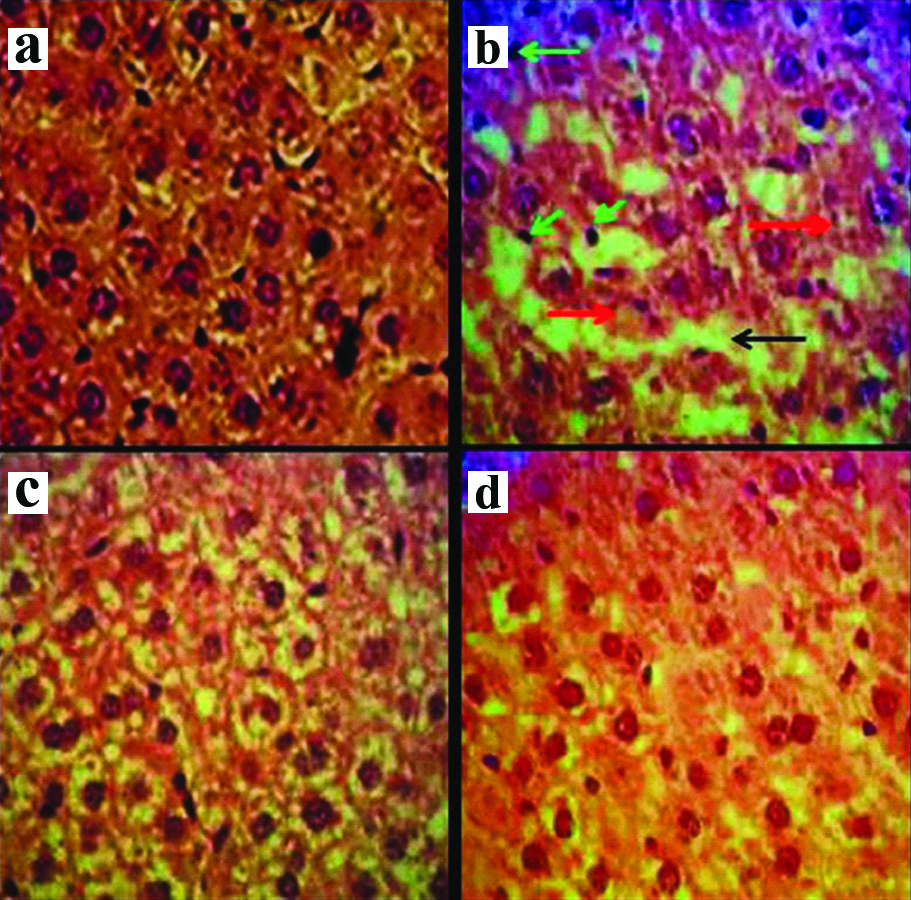
Histopathological changes: The normal structure of liver was observed in the normal control group. Increased white blood cells (Inflammation), increased irregularities and the vacuolisation hepatocyte (necrosis) and the sinusoidal dilatation was observed due to the oxidative stress caused by ethanol in the ethanol control group. The normal liver structure was detected in F. vulgaris groups. The approximately normal liver structure was observed in F. vulgaris+ethanol groups [Table/Fig-5].
Microscopic images of liver tissue in mature rats in different groups (Five-micron thick sections, H&E staining, magnification×400). Micrograph of the liver section in the control normal group: (a) normal liver structure. Micrograph of the liver section in ethanol control group; (b) Increased white blood cells (Inflammation) (green arrow), increase irregularities and the vacuolization hepatocyte (necrosis) (red arrow) and sinusoidal dilatation (black arrow), due to the oxidative stress caused by ethanol. Micrograph of the liver section in F. vulgaris (150 mg/kg) group; (c), normal liver structure. Micrograph of liver section in F. vulgaris + ethanol (150 mg/kg) group, approximately normal liver structure.
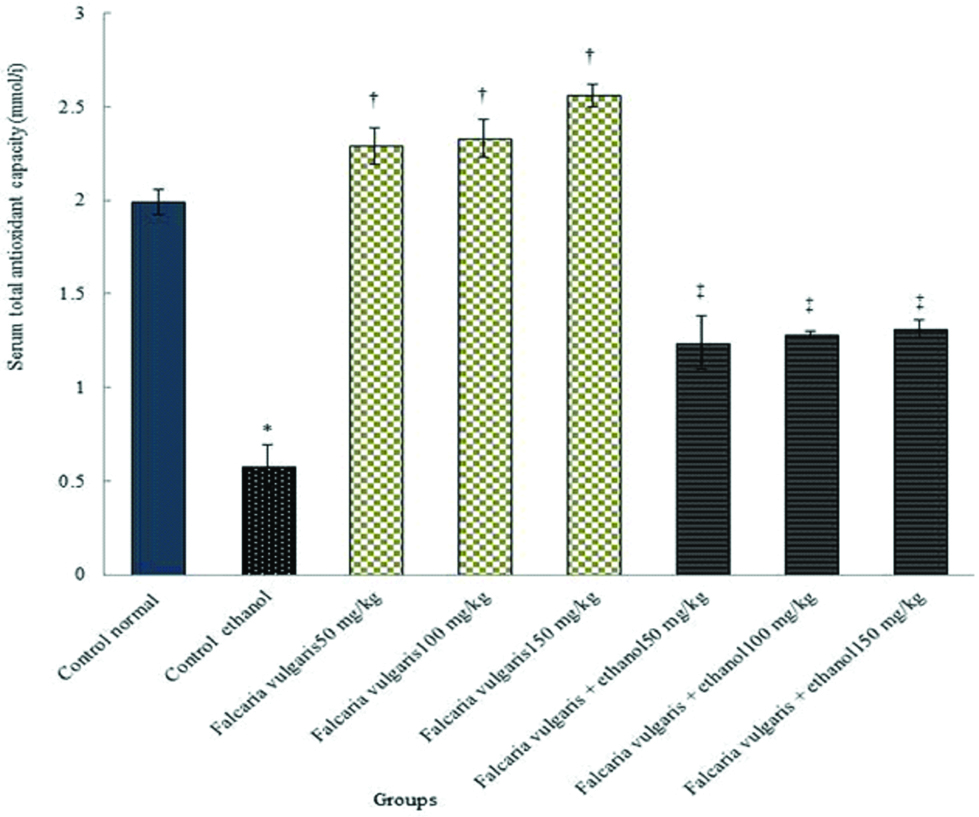
Total antioxidant capacity: The results demonstrated that the total antioxidant capacity serum level reduced significantly in the ethanol control group compared to the normal control group (p<0.05). The total antioxidant capacity level improved significantly in all F. vulgaris and F. vulgaris+ethanol groups compared to the ethanol control group (p<0.05) [Table/Fig-6].
Comparison of total antioxidant capacity in ethanol and normal control, F. vulgaris and F. vulgaris+ethanol groups. *Significant decrease in ethanol control group compared to the normal control group (p<0.05). †Significant increase for all F. vulgaris groups compared to the ethanol control group (p<0.05). ‡Significant increase for all F. vulgaris+ethanol groups compared to the ethanol control group (p<0.05).
Discussion
Ethanol is metabolised by the three systems of alcohol dehydrogenase, Cytochrome P450 and catalase in the liver. Toxic and active secondary metabolites (acetaldehyde and acetate) are produced during alcohol metabolism in the liver and activation of hepatic microsomes by the cytochrome P450 enzymes. These secondary metabolites can cause damage to various tissues, including the liver [16]. Alcohol consumption increases the cytochrome P450 capacity in the liver, due to oxidative products and the damages [17]. The aim of this study was to investigate the effect of F. vulgaris extract on liver tissue toxicity induced by ethanol. The present study demonstrates that ethanol administration increased the amount of nitric oxide level in laboratory animals significantly. Nitric oxide level decreased significantly after the animals treated with Falcaria vulgaris. It seems that the increased nitric oxide concentration, inhibits oxygen consumption, so reducing the aerobic capacity of cells, can cause damage to cells [18]. The results of a study by Szkudelski T, showed that induction of hyperglycaemia by STZ causes the destruction of islets of langerhans beta cells by oxidant production and inappropriate nitric oxide production, and this result is in line with the findings of the present study [19]. It appears that antioxidant-rich substances such as F. vulgaris can reduce the oxidative damages by reducing the free radicals and reactive oxygen species in the human body [20]. The presence of tannins and saponins in F. vulgaris can grant it antioxidant attributes [10]. The results of the study by Jang KJ et al., have indicated that saponins can inhibit iNOS activity which is consistent with the result of present study [21]. Also, the results of the study by Yang H et al., were in line with the results of the present research, suggesting that Crocin as an antioxidant causes inhibition of nitric oxide-inducing Lipopolysaccharide through inducing expression of HO-1 and Calmodulin-calcium-dependent kinase-4 protein [22]. In the current study, ethanol decreased the liver weight in ethanol control group significantly. Also, the F. vulgaris+ethanol administration groups demonstrated a significant increase in rats’ weights in contrast to the ethanol control group. The finding of Nimenibo-Uadia R showed that administration of the extract of Vernonia amygdalina (the content of alkaloids, saponins and flavonoids) to diabetic rats resulted in their recovery and gaining of lost weight. Thus, these results are in line with the findings of the present study [23]. Flavonoids seem to have a significant effect on the metabolism of carbohydrates with their ability to eliminate oxidative stress [24]. The results of the study by St-Onge MP, have indicated that sickleweed hydroalcoholic extract effectiveness on restoring weight loss as one of the most important symptoms of streptozotocin-induced diabetes which is in line with the findings of the current study [25]. Ethanol administration causes endotoxins and inflammatory agents to discharge, including interferon-γ and pre-inflammation factors like TNF-α and TGF-β and due to the liver damage [26]. The results of the present study have shown that ethanol administration can cause reductions in the number of hepatocytes and diameter of CHV. The results of the study by Mahmoud AM et al., were in line with the present study, which showed that administration of methotrexate as an oxidation agent, causes breakdown and necrosis of hepatocytes, hyperperfusion of sinusoid spaces, and significant increase in the expression of TNF-α levels [27]. A significant decrease in the number of hepatocytes and increase in the CHV diameter were recognised in the ethanol+Falcaria groups compared to the ethanol control group. It appears that oxidative stress through ethanol administration creates free radicals due to cell destruction [28]. Hydrogen peroxide and superoxide can cause cell damage as well as intracellular DNA, protein and lipid destruction which will eventually lead to the hepatic tissue lesion [29]. Zhou H et al., indicated that ethanol causes the expression of TNF-α factor and apoptosis induction in hepatocytes which agrees with the results of the current study [30]. It appears that the existence of antioxidant ingredients in F. vulgaris can protect the liver and neutralise the ethanol’s oxidation effect [10,11]. The results of the study by Biswas A et al., have shown that saponins and tannins protective hepatic cells’ and decrease liver enzymes’ in hypercholesterolemic rats which agrees with the results of the current study [31]. Also, Kasdallah-Grissa A et al., illustrated that resveratrol as an antioxidant, inhibits the induction of ethanol’s undesirable effects through the inhibition of lipid’s induction, which is in line with the findings of the current study [32]. The results of the present study demonstrated a significant increase in the AST, ALT and ALP enzymes’ levels in the ethanol control group compared to the normal control group. Moreover, a significant decrease was observed in the hepatic enzymes levels in F. vulgaris and ethanol+F. vulgaris groups compared to the ethanol control group. Increasing liver ALP, ALT and AST enzymes indicates damage to the hepatic cells [16]. Diwan SY, reported that administration of peganum harmala as an antioxidant increased serum liver enzymes in the rats treated with methotrexate, which is in line with the findings of the present study [33]. The results of Uluısık D and Keskin E, agree with the findings of the current study that Ginseng (rich in saponins) administration reduces significantly the liver enzymes in rats fed on cholesterol rich diet [34]. It seems that the hepatic cells are influenced by the alcohol intoxication and oxidative stress induced by ethanol and the cellular damage leads to the discharging of hepatic enzymes in the plasma [35]. Also, ethanol administration can activate Kupffer cells and cause the secretion of inflammatory mediators due to hepatic lesion [36]. It appears that F. vulgaris extract prevents membrane destruction and apoptosis of the hepatic cells by increasing the antioxidant capacity, reduction of nitric oxide level as well as decreasing the hepatic enzymes’ levels. The results of the current study reveal that ethanol reduced the total antioxidant capacity serum level, but the total antioxidant capacity serum level improved significantly in the F. vulgaris and F. vulgaris+ethanol groups compared to the ethanol control group. The reduction in total antioxidant capacity level in this study shows the effects of oxidative stress from ethanol on liver parameters. Ethanol induces oxidative stress in liver tissue. This demonstrates as growth in the levels of ROS and lipid peroxidation and a reduction in the action of antioxidant enzymes like total antioxidant capacity. In the present study, improved levels of total antioxidant capacity in rats treated with F. vulgaris highlight the antioxidant and anti-lipid peroxidation effects of F. vulgaris. Wang Y et al., showed that administration of ethanol, induced hepatocarcinogenesis in rats and reducing serum levels of total antioxidant capacity, which confirms the results of the present study [37]. This study revealed that F. vulgaris may be an appropriate solution to the reduction of oxidative stress damages resulting from ethanol administration.
Limitation
The present study had certain limitations; there was not much detail about the mechanism of extract action on the liver. There was a lack of references for the plant or extract. The plant or its extract was not available in the market and authors had to wait until the planting season (spring). Financial problems for performing techniques and death of some animals due to alcohol administration in this study are the most important limitations. Hence prospective studies should be taken for detailed association of the molecular interaction between F. vulgaris and ethanol.
Conclusion
The findings of this study showed that F. vulgaris hydroalcoholic extract can advance some of the liver damages against the toxic effects of ethanol in rats. The results also suggest the potential effects of F. vulgaris especially antioxidant effects against toxic effects of ethanol. F. vulgaris may offer a novel therapeutic approach. However, further research on animal models is necessary to obtain more conclusive evidence for the molecular interaction between F. vulgaris and ethanol leading to advance hepatic damage.