Infective Endocarditis (IE) remains a condition associated with high morbidity and mortality rates and associated with poor prognosis despite improvements in medical and surgical therapies and remains a challenging entity [1]. Main threat associated with endocarditis comprises the increasing populace of adults with congenital heart disease and patients with repeated healthcare interaction for other comorbidities. Besides, IE include patients who are haemodialysed and immunocompromised or injecting drugs [2]. Although, IE may present with several very different initial symptoms, its diagnosis usually relies on the association of clinical, microbiological and morphological criteria, which are included in the modified Duke classification [3].
IE is primarily a disease of young patients with Rheumatic Heart Disease (RHD) in low to middle-income countries and carries a very poor prognosis [4]. In Africa, there is lack of data where rheumatic valvular heart disease is still highly prevalent. It makes decisions regarding the optimal treatment of this disease very difficult. To make matters worse, there has been considerable controversy surrounding prevention of the disease [5].
Echocardiography is the primary imaging modality for diagnosis of endocarditis. It plays a key role not only in the diagnosis of IE but also in the prognostic assessment and follow-up under therapy and during surgery [6]. Recommendations for the practice of echocardiography in IE have been published. It provides an update on the value and limitations of this technique in IE. It defines the optimal use of Transthoracic Echocardiography (TTE) and Trans-Oesophageal Echocardiography (TEE) [2]. The diagnostic report of TTE are mainly derived from studies published before the 2000s [7-9] and from case-control studies [10-12]. Nevertheless, the progress in echocardiographic and imaging technology in the last 15 years could have an effect on the diagnostic correctness of TTE, particularly for native valve endocarditis. Instead, the achievement of optimal image quality depends on patients’ characteristics. Consequently, TEE is supported as reference standard in categorising vegetation, but then again has the drawback of being reasonably invasive and resource consuming in contrast to TTE [7].
In this prospective quantitative observational study, patients with IE were evaluated to correlate the echocardiographic findings with the intraoperative findings. As a secondary goal, we aimed to assess the severity of complications in patients with IE and compare blood cultures to echocardiographic findings.
Materials and Methods
The study design was a prospective, quantitative and observational, conducted on 40 patients with IE, at Dr George Mukhari Hospital (DGMH), Pretoria, South Africa. The sample size was determined using software EPi-Info version 3.5 with a 95% confidence interval and a frequency of 50%. A sample of 40 was obtained from population of 45. All 40 patients gave written informed consent to participate in the study, which was approved by the Institutional Research Ethics Committee of Durban University of Technology, Durban.
Infective endocarditis was diagnosed according to Duke criteria of 1994 [8,9]. Since the beginning of the study, all historical, clinical, laboratory, echocardiographic and microbiologic data were entered in a database specifically designed for this investigation.
Inclusion Criteria
Male and female patients aged 20-45 years with infective endocarditis in the aortic, mitral or tricuspid valve requiring surgical intervention and who were willing to participate in the study and provided written informed consent form were included in the study.
Exclusion Criteria
Cardiac patients with congenital heart diseases (e.g., ventricular septal defects, patent ductus arteriosus, atrial septal defects) or critically ill/unstable patients with infective endocarditis of non-bacterial origin (e.g., myxoma, viral and fungal) were excluded from study.
Data Collection
Step 1: Patient identification
Patients were identified through the referral system and incidentally by echocardiography. A physician (cardiologist) examined patients clinically for features of IE, which included splinter haemorrhages, conjunctival petechiae, Osler’s nodes and Janeway’s lesions.
Step 2: Echocardiography
The echocardiographic examinations were done with commercially available instruments (Hitachi Aloka). All patients were subjected to both modalities of echocardiographic imaging-TTE and TEE.
Step 3: Echocardiographical measurements of the mitral valve and severe mitral regurgitation in patients with IE.
Step 4: Data recording for echocardiogram findings, clinical diagnosis before the operation and intraoperative findings (visual and histology) for patients with IE.
Step 5: Culture analysis
Step 6: Performance of surgery (valve replacements) and assessment of intraoperative findings by TEE.
Ethics Statement
This study was approved by the Ethical Committee of the Durban University of Technology (Project Reference number 45/12) and the Research Committee, Dr George Mukhari Hospital, Pretoria, South Africa and was performed in accordance with the Helsinki Declaration of 1975 (as revised in 1983).
Statistical Analysis
SPSS version 15.0 (SPSS Inc., Chicago, Illinois, USA) was used for analysis of data. A p-value of <0.05 was considered as statistically significant. Clinical data were compared between the intervention and control groups using Mann-Whitney tests for non-parametrically distributed dependent variables, t-tests for those which were normally distributed, and Pearson’s chi-square tests for categorical variables.
Results
Demographics and Baseline Characteristics
Forty patients (n=40) were recruited in the study. Their mean age±standard deviation for echocardiography and intraoperative findings was 31.7±7.6 years and their ages ranged from 20 to 45 years. [Table/Fig-1] shows descriptive statistics of age. No significant statistical difference was observed between echocardiograph and intraoperative findings for LVED (p>0.05). This was confirmed by a moderate correlation/association of (r=0.82). There were 19 of 25 (76%) male patients with IE on mitral valve and aortic valve; 10 of 15 (66%) female patients with IE on the mitral valve and aortic valve. Male patients with IE on tricuspid valve were 6 of 25 (24%); 5 of 15 (33%) female patients with IE on tricuspid valve.
Comparative analysis of variables of echocardiography and intraoperative findings.
| Variables | Echocardiography | Intraoperative findings | p-value | r-value |
|---|
| Mean±SD | Range | Mean±SD | Range |
|---|
| Age | 31.2±7.61 | 20-45 | 31.73±7.61 | 20-45 | p>0.05 | r=1.0 |
| LVED* | 53.8±8.65 | 38-80 | 53.43±10.24 | 39-70 | p>0.05 | r=0.819 |
| LVES* | 34.97±8.58 | 19-59 | 34.12±9.62 | 19-50 | p>0.05 | r=0.677 |
| EF* | 59.54±11.59 | 21-78 | 60.56±15.67 | 19-78 | p>0.05 | r=0.876 |
| SF* | 31.36±7.14 | 10-45 | 32.8±10.11 | 8-45 | p>0.05 | r=0.798 |
*LVED: Left ventricle end-diastolic diameter; LVES: Left ventricle end-systolic diameter; EF: Ejection fraction; SF: Shortening fraction
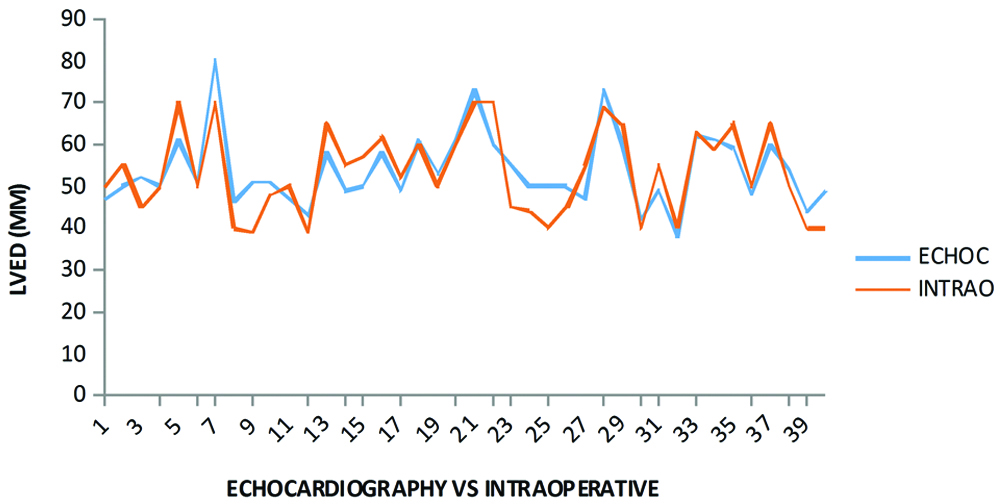
Correlation for LVED of Echocardiography and Intraoperative Findings
No significant statistical difference was observed between echocardiograph and intraoperative findings for LVED (p>0.05) as shown in [Table/Fig-2] {moderate correlation/association of (r=0.82)}.
LVED of the echocardiography in competition with intraoperative findings.
Clinical and Microbiological Data
[Table/Fig-3] represents clinical diagnosis of all patients (n=40). The intraoperative and echocardiography findings showed 32 of 40 (80%) vegetation, 2 of 40 (5%) perforation, 4 of 40 (10%) pseudo-aneurysm and 2 of 40 (5%) abscesses. The prognosis of patients with poor ejection fraction (40-50% EF) was poorer than those with good ejection fraction (60-75%). The clinical findings of all patients confirmed infective endocarditis and 32 of 40 (80%) blood cultures were positive and 8 of 40 (20%) were negative. There were 7 of 40 (17.5%) patients who showed poor correlation 40-50% between echocardiographical findings and postoperative findings. The results of 33 of 40 (82%) patients showed perfect correlation 69% between the echocardiographical findings and postoperative findings [10]. It was noticed, 8 of 40 (20%) had stenosis and 32 of 40 (80%) had regurgitation in patients who had infective endocarditis.
Clinical diagnosis of infective endocarditis patients.
| Clinical diagnosis | Percentages (%) | Number of patients |
|---|
| Anaemia | 40% | 16 |
| Fever | 90% | 36 |
| Finger clubbing | 40% | 16 |
| Splenomegaly | 40% | 16 |
| Changing or new murmurs | 85% | 34 |
| Evidence of cardiac embolism | 40% | 16 |
Discussion
The incidence of IE in the developed world is rising and is intrinsically linked to healthcare provision for an ageing and susceptible population [5]. The male to female case ratio is more than 2:1 [3]. The highest rates were observed among patients with prosthetic valves, intracardiac devices, unrepaired cyanotic congenital heart diseases, or a history of IE. Although 50% of cases of IE develop in patients with no known history of valve disease [9]. In the present study, 25 of 40 patients (61%) were males and 15 of 40 patients (39%) were females. The results of the study indicate that the IE occurred at the age range from 20-45 (p> 0.05, r =1.0) [Table/Fig-1]. This may provide vital information to clinicians to assist in reducing the death rate of patients.
This study found that 40% of patients with splenomegaly were fine for more than six months. Within the clinical findings, 90% of patients (n=36, n=34) had fever and 85% had changing or new murmurs [Table/Fig-3]. Thirty-two of 40 patients (80%) were culture positive and 8 of 40 patients (20%) were culture negative. Andrews MM et al., has concluded that positive blood cultures are a major diagnostic criterion for IE and key to identifying the aetiologic agent and the optimal antimicrobial regimen [11].
In the present study, the intraoperative and echocardiography findings presented presence of vegetation in 80% of the patients, the presence of perforation in 5% of patients, pseudo-aneurysm in 10% of patients and the presence of abscess in 5% of patients. Vilacosta I et al., demonstrated that the increase risk of embolisation also increases vegetation size [12]. This is particularly significant in mitral endocarditis and staphylococcal endocarditis. An increase in vegetation size, despite antimicrobial treatment, may predict later embolism. In our study, preoperative findings confirmed 24 of 40 (60%) streptococci, 8 of 40 (20%) enterococci, 6 of 40 (15%) mixture of organisms and 2 of 40 (5%) Gram-negative bacilli. The role of echocardiography in predicting embolic complications is controversial [13]. Mügge A et al., stated that when the infection involved a native aortic or prosthetic valve (in either the aortic or mitral position), the size of the vegetation was not significantly different between episodes involving embolism and those not involving embolism [14]. However, among patients with an infected native mitral valve, those with a large vegetation (>10 mm) had a higher incidence of embolism than those with a small vegetation. The prediction of individual patient risk for embolisation is extremely difficult. Many have attempted to use echocardiography to identify a high-risk subset of IE patients who might benefit from early surgery to avoid embolisation [15].
Echocardiography performed in the present study presented a preserved left ventricular systolic function (EF>50%) in 80% of patients. In 20%, left ventricular function was moderately depressed (EF between 35 and 50%). Overall, moderate to severe valvular regurgitation occurred in 80% of patients with mitral and aortic involvement and 20% of patients with stenosis of both valves. The findings are in agreement with Kim N et al., on the basis of study results the authors recommended that operative risk associated with multiple valve involvement should not exceed the risk of single valve disease, irrespective of the affected valve type [16].
In the present study, there were no surgeries and persistent infections nonetheless all patients being in active phase of IE. This demonstrates the success with the radical approach in this institution. It did result in the onset of complete atrioventricular block postoperatively in two patients necessitating pacemaker implantation.
Conclusion
There was no significant difference between the echocardiography and intraoperative findings for LVED (p>0.05). Further, it was identified that 20% of the study IE participants had stenosis and 32 (80%) had regurgitation. The medical and surgical treatment decision can be guided by echocardiographic detection of fistulae, prosthetic dehiscence, obstructive vegetation or flail leaflets.
Limitation
The study was limited due to time constrains and budget allocation as the study was limited to one hospital.
*LVED: Left ventricle end-diastolic diameter; LVES: Left ventricle end-systolic diameter; EF: Ejection fraction; SF: Shortening fraction