Haemolytic Uremic Syndrome (HUS) and Posterior Reversible Encephalopathy Syndrome (PRES) are two diverse conditions that may have common triggering pathways. Postpartum HUS is a known phenomenon that complicates pregnancy with eclampsia and haemolysis, elevated liver enzymes and low platelet count (HELLP syndrome). Treatment is usually by plasmapheresis and heamodialysis. PRES, though rare may complicate pregnancy with eclampsia. We present a case of a 24-year-old primigravida who developed postpartum HUS and PRES simultaneously making it a rarest combination.
Eclampsia, Haemodialysis, Plasmapheresis, Primigravida
Case Report
A 24 years old patient G1P0L0, house wife, teli by caste, Hindu by religion, presented to the obstetric clinic of our hospital in labor at 39 weeks of gestation. She was an unbooked case, without any prenatal visits. There was no prior history of fever and abnormal bleeding and diarrhea, vomiting and oliguria. She was non-hypertensive and nondiabetic. On examination she was having tachycardia, blood pressure 220/118 mm Hg, facial puffiness and mild pitting oedema was present in bilateral lower limbs. JVP was normal. Cardiovascular and Respiratory system examination did not reveal any abnormalities.
The patient was diagnosed to have pre-eclampsia in view of hypertension, and a urine sample showed proteinuria and she underwent Lower Section Cesarean Section (LSCS). The cesarean section was uneventful with delivery of male infant. Forty-eight hours after delivery, the patient complained of palpitation and oliguria. Examination revealed height of 162 cm, weight 54 kg, BMI-20.6 Kg/m2, pulse of 140 beats per minute, regular, severe pallor was present, mild icterus was present and Jugular Venous Pulse (JVP) was normal. The laboratory investigations at that time revealed haemoglobin (Hb) 4 gm/dL, MCV 66 fl, Red cell distribution width 18, serum LDH 6693 mg/dL, absolute platelet count 24000/cumm. The renal function test showed serum urea of 112 mg/dL, serum creatinine 6.81 mg/dL, serum potassium 5.8 mEq/L, serum sodium 130 mEq/L, serum calcium 8 mg/dL. The peripheral smear revealed schistocytes. Coagulation profile was normal, Liver Function Test (LFT), Aspartate transaminase (AST) 60 IU/L, Alanine Transaminases (ALT) 70 IU/L, indirect bilirubin was raised. Coomb’s test was negative. ELISA for HIV was negative. In view of schistocytes in the peripheral smear, rising uremia and thrombocytopenia, possibility of HUS and Haemolysis Elevated Liver Enzymes and Low Platelet count (HELLP) was entertained. Further investigations were ordered. Antinuclear Antibody (ANA), ANTI-dsDNA, complement factor C3 and C4, antibody to C5b, Anti Phospholipids Antibody (APLA), compliment H and I antibodies were negative. A blood ADAMTS 13 activity was 7% (normal range 66–126%).
The patient was treated with Tab. Nicardipine 20 Mg TID, Tab. Metoprolol 25 OD. Final diagnosis of Postpartum haemolytic uremic syndrome was established and patient was treated with haemodialysis and plasmapheresis. The patient underwent eight sessions of haemodialysis over the period of two weeks and eight sessions of plasma exchange over two weeks. [Table/Fig-1] summarises the clinical course of HUS in the patient.
Clinical profile of the patient.
| LDH in mg% | Serum urea in mg % | Serum creatinine | AST in IU/L | Platelet count/cumm | Urine output in 24 hours |
|---|
| Day 2 (postoperative) | 6693 | 144 | 6.81 | 212 | 44,000 | Nil |
| Day 5 | 2884 | 128 | 6.71 | 71 | 89,000 | Nil |
| Day 10 | 2044 | 103 | 5.75 | 59 | 1,12,000 | 30 mL |
| Day 15 | 1000 | 68 | 3.9 | 43 | 2,52,000 | 230 mL |
| Day 21 | 450 | 36 | 1.11 | 22 | 3,43,000 | 700 mL |
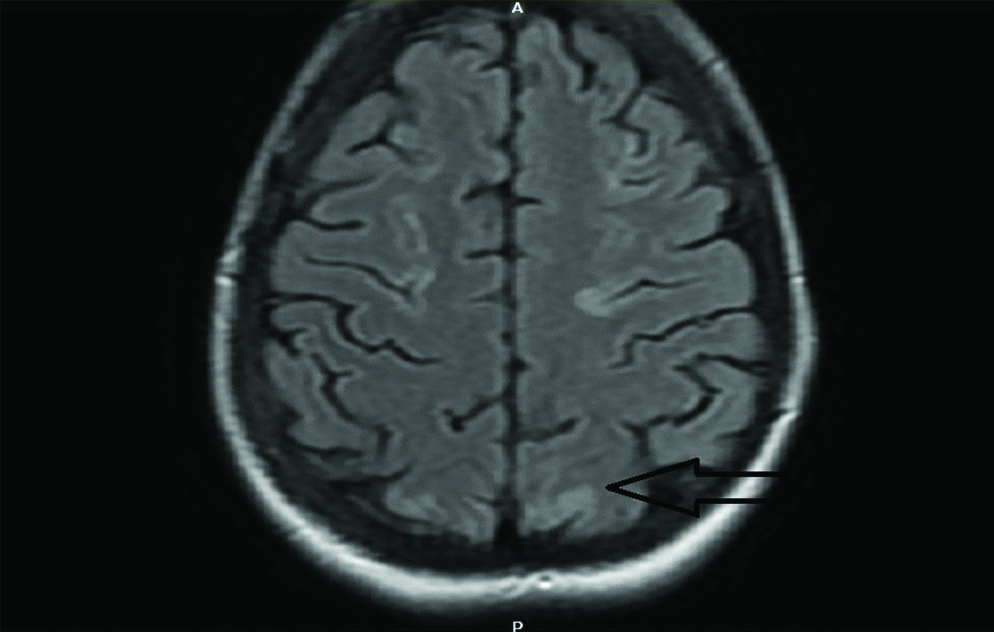
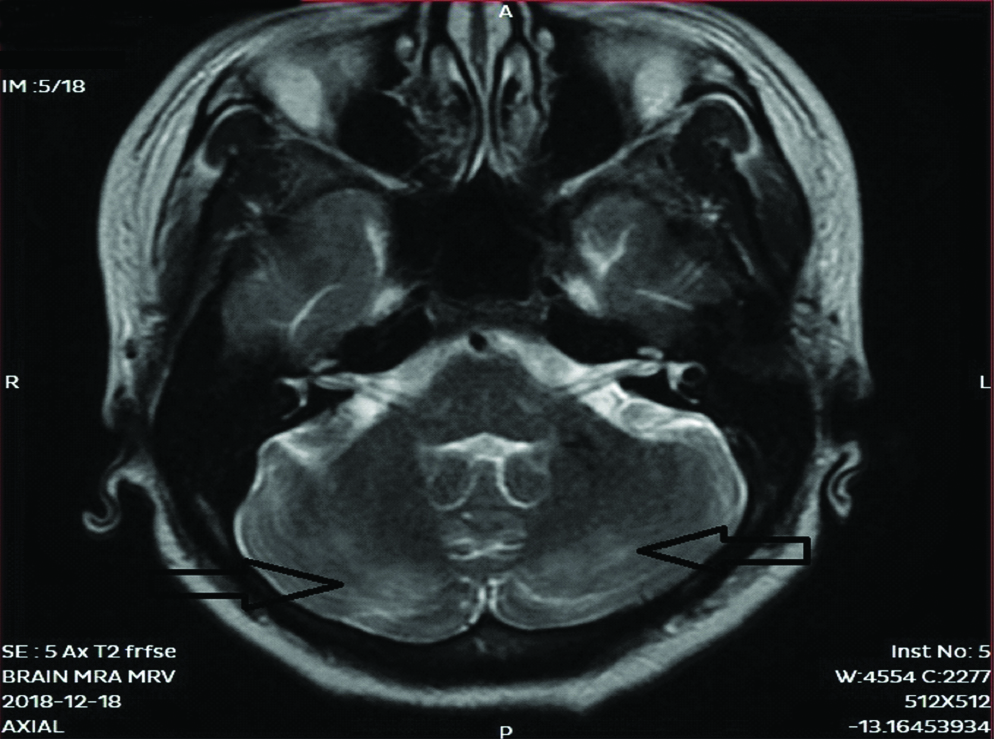
On 8th day of treatment session, patient complained of sudden onset of severe headache which was associated with two episodes of vomiting. The intensity of headache increased over the next two hours and subsequently patient complained of blurring of vision and the patient had one episode of Generalized Tonic Clonic Seizures (GTCS) which lasted for 30 seconds. In view of these neurological complications, an urgent MRI was done which revealed cortical and subcortical T2 and FLAIR white matter hyperintensities noted in bilateral frontal, parietal-occipital and cerebellar regions suggestive of Posterior Reversible Encephalopathy Syndrome (PRES) [Table/Fig-2,3].
MRI brain in T2 FLAIR scan showing hyperintensities in occipital lobe.
MRI Brain T2 FR-FSE with representing hyperintensities in the cerebellum.
The patient was then started with injection dexamethasone 4 mg IV 6 hourly and IV Mannitol 2 doses 12 hours apart and IV levetiracetam 500 mg twice daily for seizures. The headache decreased over next two days and repeat MRI after five days was normal.
Intermittent haemodialysis and intermittent plasmapheresis was continued for 1 more week. Kidney Function Test (KFT) and urine output all were improved and patient was discharged. Follow-up after one week urea, creatinine and urine output was found to be normal.
Discussion
Postpartum Haemolytic Uremic Syndrome (PHUS) is a rare, severe form of Thrombotic Microangiopathy (TMA) characterized by Acute Kidney Injury (AKI) which usually occurs immediately after delivery to 10 weeks postpartum. The clinical triad of HUS is Microangiopathic Haemolytic Anemia (MAHA), thrombocytopenia, and acute renal failure [1]. It usually occurs in primigravida with the mean age of 27.0±6 years and is usually associated with pre-eclampsia [2]. Typical HUS is due to damage to the endothelial cells which can result from following pathogenic mechanisms: Verotoxin-induced endothelial cell activation and apoptosis in Shiga toxin-induced HUS. Atypical HUS (a HUS) is due to acquired or constitutional complement alternative pathway dysregulation leading to complement-induced endothelial cell damage. Pregnancy carries a high risk for various forms of thrombotic microangiopathy, including ADAMTS13 deficiency associated thrombotic thrombocytopenic purpura but also HUS [3].
Pregnancy-associated HUS is a form of secondary HUS. The pathophysiologic basis of secondary forms of HUS is due to alternate complement pathway dysregulation combined with specific precipitating events. The management protocol involves supportive care, Plasma exchange and Eculizumab, a monoclonal antibody to C5 that blocks the terminal complement cascade [4].
The peripheral smear typically shows schistocytes which is a hallmark of haemolysis along with increased Lactate Dehydrogenase (LDH) level. The basic classification of HUS consists of Shiga toxin producing Escherichia coli infection (STEC-HUS), atypical HUS, which is thought to be of mutation in the gene coding for complement regulators in plasma, complement factor H [5] and lastly secondary HUS where it is caused by co-existing diseases like Autoimmunity disorders [6], solid organ transplantation [7], systemic malignancies [8], septicemia and pregnancy [9], pre-eclampsia, HELLP syndrome [10], or with cytotoxic drugs [11,12].
Pre-eclampsia and HELLP are one of the known triggers for postpartum HUS and, it is associated with 15% cases [2]. The estimated incidence of postpartum HUS is around 1/25000 pregnancies [13]. It can be triggered by abruptio placentae, spontaneous abortions, Anti-Phospholipid antibody syndrome, pregnancy-induced hypertension [14-16]. These conditions increases procoagulant factors in the blood and decreases the fibrinolytic activity in the blood and leads to thrombotic microangiopathic anaemia [17]. Some literature suggest that the renal failure in postpartum HUS is because of a platelet aggregating factor which causes deposition of micro thrombin in vessel walls occluding the microvasculature of kidney leading to AKI [18]. Supporting evidences for the confirmation of a HUS sometimes require biopsy of the kidney which reveals thrombotic miroangiopathy in the small intra-renal vessels. Decrease of complement I levels also supports the diagnosis of postpartum HUS. Patient did not consent for biopsy in our case. Recent evidences suggest thrombotic microangiopathies in HUS may also occur due to ultra-large von Willebrand factor (UL-VWF)-platelet thrombi formations in the circulation because of deficiency of a VWF cleavage protein ADMST 13 [9]. Our patient also had a severe deficiency of ADMST 13 (7%). [Table/Fig-4] enlists the probable risk factors for development of HUS in our case.
Probable risk factors causing HUS in our patient.
| Risk factor present | Risk factor absent |
|---|
| 1 | Pregnancy | 1 | APLA negative |
| 2 | Primigravida | 2 | Complement factor H negative (confirming absence of congenital HUS) |
| 3 | Pre-eclampsia | 3 | Anti-nuclear antibody (ANA), Anti-ds DNA antibodies, compliment C3 C4 negative(ruling out autoimmune condition) |
| 4 | HELLP syndrome | 4 | No history of diarrhoea (Presumptive negativity of E.coli) |
| 5 | Hypertension | |
| 6 | ADMST 13 <10% |
PRES is a syndrome characterised by a headache, seizures, altered mental status and visual loss. The pathology is usually a vasogenic oedema predominantly in the posterior occipital and parietal lobes of the brain. The usual predisposing factors are pre-eclampsia/eclampsia, allogeneic bone marrow transplantation, organ transplantation, autoimmune disease and chemotherapy [19,20].
Hypertension, pre-eclampsia, pregnancy and HUS are conditions that may accompany PRES [21]. Our patient was having HELLP with severe hypertension. Another factor in our case that might have triggered development of PRES was the sessions of haemodialysis. ‘Dialysis Disequilibrium Syndrome’ (DDS) is a known entity which occurs in cases of severe uremia undergoing acute dialysis, which causes cerebral oedema because of intracerebral osmotic shifts. DDS may rarely present as PRES. The post dialysis clinical symptoms of DDS were present in our case [22].
Hypertension induces cerebral vasospasm leading to ischemia and cytotoxic oedema in the brain. Another hypothesis suggests that PRES occurs due to failure of cerebral autoregulation leading to cerebral arteriolar vasodilatation which causes vasogenic oedema [23].
The choice of imaging modality for the diagnosis of PRES is MRI Flair Sequence, which shows abnormal T2 weighted image densities in the parieto-occipital region, cerebellum and rarely other areas: parietal-occipital and cerebellar regions. Our patient had suggestive findings in parietal-occipital and cerebellar regions. The vertebrobasilar system is less innervated by sympathetic nerves as compared to anterior circulation so autoregulation failure occurs in posterior circulation leading to characteristic MRI findings in parieto occipital and brain stem areas [24]. PRES is usually reversible if the inciting events are identified and treated early.
Conclusion
Ours is a rare case of a primigravida with pre-eclampsia complicating into secondary HUS and PRES. It is imperative for clinicians/obstetricians to keep in mind rare presentations like these which can be triggered by common obstetric conditions like pre-eclampsia, HELLP. All cases of eclampsia should be astutely evaluated for the renal profile, hematological profiles while keeping these complications in mind. Earlier intervention usually leads to a positive outcome.
[1]. Gasser C, Gautier E, Steck A, Siebenmann R, Oechslin R, Hemolytic-uremic syndrome: Bilateral necrosis of the renal cortex in acute acquired hemolytic anaemiaSchweiz Med Wochenschr 1955 85(38-39):905-09. [Google Scholar]
[2]. Weiner CP, Thrombotic microangiopathy in pregnancy and the postpartum periodSemin Hematol 1987 24(4):271-76. [Google Scholar]
[3]. Scully M, Goodship T, How I treat thrombotic thrombocytopenic purpura and atypical haemolytic uraemic syndromeBr J Haematol 2014 164(6):759-66.10.1111/bjh.1271824387053 [Google Scholar] [CrossRef] [PubMed]
[4]. Bruel A, Kavanagh D, Noris M, Delmas Y, Wong E, Bresin E, Hemolytic uremic syndrome in pregnancy and postpartumClin J Am Soc Nephrol 2017 12(8):1237-47.10.2215/CJN.0028011728596415 [Google Scholar] [CrossRef] [PubMed]
[5]. Warwicker P, Goodship TH, Donne RL, Pirson Y, Nicholls A, Ward RM, Genetic studies into inherited and sporadic hemolytic uremic syndromeKidney Int 1998 53(4):836-44.10.1111/j.1523-1755.1998.00824.x9551389 [Google Scholar] [CrossRef] [PubMed]
[6]. Song D, Wu LH, Wang FM, Yang XW, Zhu D, Chen M, The spectrum of renal thrombotic microangiopathy in lupus nephritisArthritis Res Ther 2013 15(1):R1210.1186/ar414223320601 [Google Scholar] [CrossRef] [PubMed]
[7]. Caires RA, Marques ID, Repizo LP, Sato VA, Carmo LP, Machado DJ, De novo thrombotic microangiopathy after kidney transplantation: Clinical features, treatment, and long-term patient and graft survivalTransplant Proc 2012 44(8):2388-90.10.1016/j.transproceed.2012.07.03923026601 [Google Scholar] [CrossRef] [PubMed]
[8]. Lechner K, Obermeier HL, Cancer-related microangiopathic hemolytic anaemia: Clinical and laboratory features in 168 reported casesMedicine (Baltimore) 2012 91(4):195-205.10.1097/MD.0b013e318260359822732949 [Google Scholar] [CrossRef] [PubMed]
[9]. Egbor M, Johnson A, Harris F, Makanjoula D, Shehata H, Pregnancy-associated atypical haemolyticuraemic syndrome in the postpartum period: A case report and review of the literatureObstet Med 2011 4(2):83-85.10.1258/om.2011.10005927582861 [Google Scholar] [CrossRef] [PubMed]
[10]. Tufano A, Coppola A, Maruotti GM, Martinelli P, Cerbone AM, Di Minno G, HELLP syndrome and its relation with the antiphospholipid syndromeBlood Transfus 2014 12(1):114-18. [Google Scholar]
[11]. Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, An international consensus approach to the management of atypical hemolytic uremic syndrome in childrenPediatr Nephrol 2016 31(1):15-39.10.1007/s00467-015-3076-825859752 [Google Scholar] [CrossRef] [PubMed]
[12]. Mele C, Remuzzi G, Noris M, Hemolytic uremic syndromeSemin Immunopathol 2014 36(4):399-420.10.1007/s00281-014-0416-x24526222 [Google Scholar] [CrossRef] [PubMed]
[13]. Dashe JS, Ramin SM, Cunnigham FG, The long-term consequences of thrombotic microangiopathy (thrombotic thrombocytopenic purpura and hemolytic uremic syndrome) in pregnancyObstet Gynecol 1998 91(5 Pt 1):662-68.10.1097/00006250-199805000-000049572207 [Google Scholar] [CrossRef] [PubMed]
[14]. Ribeiro FM, Rocha E, Maccariello E, Caldas ML, Gomes MV, Lugon JR, Early gestational hemolytic uremic syndrome: Case report and review of literatureRen Fail 1997 19:475-79.10.3109/088602297090477339154664 [Google Scholar] [CrossRef] [PubMed]
[15]. Huang JJ, Chen MW, Sung JM, Lan RR, Wang MC, Chen F, Postpartum haemolyticuraemic syndrome associated with antiphospholipid antibodyNephrology Dialysis Transplantation 1998 13:182-86.10.1093/ndt/13.1.1829481737 [Google Scholar] [CrossRef] [PubMed]
[16]. Kniaz D, Eisenberg GM, Elrad H, Johnson CA, Valaitis J, Bregman H, Postpartum hemolytic uremic syndrome associated with antiphospholipid antibodies: A case report and review of the literatureAmer J Nephrol 1992 12:126-33.10.1159/0001684321492877 [Google Scholar] [CrossRef] [PubMed]
[17]. Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E, Changes in health and disease of the metalloprotease that cleaves Von Willebrand factorBlood 2001 98:2730-35.10.1182/blood.V98.9.273011675345 [Google Scholar] [CrossRef] [PubMed]
[18]. Furlan M, Lammle B, Aetiology and pathogenesis of thrombotic thrombocytopenic purpura and haemolyticuraemic syndrome: The role of von Willebrand factor-cleaving proteaseBest Pract Res Clin Haematol 2001 14:437-54.10.1053/beha.2001.014211686108 [Google Scholar] [CrossRef] [PubMed]
[19]. McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findingsAJR Am J Roentgenol 2007 189(4):904-12.10.2214/AJR.07.202417885064 [Google Scholar] [CrossRef] [PubMed]
[20]. Roth C, Ferbert A, The posterior reversible encephalopathy syndrome: what’s certain, what’s new?Pract Neurol 2011 11(3):136-44.10.1136/practneurol-2011-00001021551107 [Google Scholar] [CrossRef] [PubMed]
[21]. Garg RK, Posterior leukoencephalopathy syndromePostgrad Med J 2001 77(903):24-28.10.1136/pmj.77.903.2411123390 [Google Scholar] [CrossRef] [PubMed]
[22]. Sengupta P, Biswas S, Dialysis disequilibrium leading to posterior reversible encephalopathy syndrome in chronic renal failureCEN Case Rep 2016 5(2):154-57.10.1007/s13730-016-0215-428508968 [Google Scholar] [CrossRef] [PubMed]
[23]. Vaughan CJ, Delanty N, Hypertensive emergenciesLancet 2000 356(9277):411-17.10.1016/S0140-6736(00)02539-3 [Google Scholar] [CrossRef]
[24]. Patel N, Dalal P, Panesar M, Dialysis disequilibrium syndrome: A narrative reviewSemin Dial 2008 21(5):493-98.10.1111/j.1525-139X.2008.00474.x18764799 [Google Scholar] [CrossRef] [PubMed]