Introduction
External Beam Radiotherapy (EBRT) with concurrent weekly cisplatin followed by brachytherapy boost is the standard treatment of bulky and locally advanced cervical cancer [1-3]. Four field box techniques has been used for a long time to deliver EBRT, however, when used along with concurrent cisplatin the acute Haematological Toxicity (HT) increases [1,3,4]. Studies have shown that the volume of bone marrow included in the radiotherapy fields receiving 10 and 20 Gy is associated with acute HT in patients being treated with IMRT concurrent with cisplatin [5,6]. Klopp AH et al., (RTOG 0418) showed that PBM sparing reduced HT in patients receiving pelvic RT concurrent with cisplatin [7]. They showed that V40 was a better predictor of developing ≥grade 2 HT. The highly conformal techniques of IMRT/VMAT has a potential of delivering radiation dose while avoiding surrounding normal tissues and its benefits have been proven in many anatomical sites like head and neck cancer [8,9] or prostate cancer [10,11]. Many studies have shown that IMRT reduces the dose to normal tissues for whole pelvic RT also [12-15]. Murakami N et al., have dosimetrically shown in postoperative patients of carcinoma cervix that BMS-IMRT significantly reduces the dose to the PBM [16]. The purpose of the present study was, therefore, to see whether the PBM sparing was feasible with VMAT in intact carcinoma cervix patients without compromising V95% of PTV.
Materials and Methods
The present study was a retrospective Dosimetric study conducted on 10 consecutive patients of biopsy-proven invasive cervical cancer attending the outpatient department of Apex Hospital Cancer Institute, Varanasi, Uttar Pradesh, India, between September 2017 and December 2017. As per FIGO [17] staging six patients were stage IIB, two patients were IIIA and two patients were IIIB [Table/Fig-1]. All patients were asked to come empty stomach on the day of simulation and were given two tablets of bisacodyl a night before the Radiotherapy Treatment Planning (RTP) CT scan. All patients underwent CT-based planning in a supine position and customised thermoplastic pelvic cast was made for all for the purpose of immobilisation. Patients were asked to void urine and then drink 1000 mL of water 30 minutes prior to the CT- RTP scan on the day of simulation and this bladder filling protocol before each fraction was followed strictly for the entire duration of treatment to limit interfraction and intrafraction variability. A radio-opaque vaginal marker was placed at the introitus or the lowest extent of disease as felt per vaginally on palpation. Images were obtained from T12 to the middle third of femur after administering intravenous contrast of 100 mL of omnipaque. All patient’s images were acquired using 2 mm slice thickness. Thereafter contouring was done as per the standard guidelines [18-23]. The Gross Target Volume (GTV), Clinical Target Volume (CTV1 and CTV3) and organs at risk were contoured on each axial CT slices. GTV consisted of uterus, cervix, vagina and bilateral ovaries and was contoured 3 cm below the vaginal marker or inferior border of obturator foramen depending on the lowest extent of disease. Internal Target Volume (ITV) around the GTV (anteroposteriorly and superoinferiorly 1.5 cm and laterally 7 mm) was created to take in to account the change in shape of included structures with respect to bladder filling. CTV1 comprised of nodal CTV and included, involved nodes and relevant draining nodal groups i.e., common iliac, internal iliac, external iliac, obturator and presacral Lymph Nodes (LN) and was contoured according to Taylor’s guidelines [18,19]. The upper border of nodal CTV was maintained at the bifurcation of the aorta after the uniform margin of 7 mm around the blood vessels. The bone and psoas muscles were excluded from the nodal CTV but the space between the lateral surface of a vertebral body and iliopsoas muscle was included at the level of common iliac vessels. Inguinal lymph nodes were included in cases of lower vaginal involvement. CTV3 comprised of bilateral parametrium which included the connective tissue extending from the cervix to the lateral pelvic wall. GTV and CTV3 were added together to form primary CTV. Total CTV included CTV1 and primary CTV. A uniform expansion of 10 mm was given around total CTV to form total PTV (planning target volume) to take care of daily set-up uncertainties. Eventually, final PTV was created combining total PTV and ITV. A dose of 50 Gy in 25 fractions was delivered at 2 Gy per fraction over five weeks with weekly cisplatin at 40 mg/m2 [24].
Patient’s characteristics (n=10).
| Median age (range) | 50 (38-55) |
| Clinical stage |
| IIB | 6 |
| IIIA | 2 |
| IIIB | 2 |
| Histology |
| Squamous cell carcinoma | 10 |
| Parametrium invasion present | 10 |
Normal Tissue Definition
Normal tissues delineated were bowel bag, bladder, rectum, femoral heads which were contoured as per the RTOG normal tissue guidelines [25]. Instead of individual bowel loops, bowel bag was contoured starting 1 cm above the PTV and continued to the most inferior extent in the pelvis. An outer wall of rectum was contoured on each axial slice starting from rectosigmoid junction to the anal verge and similarly, urinary bladder was contoured on each axial slice. We contoured the PBM starting from 1 cm above the superior most CT slice containing PTV to the inferior border of the ischial tuberosity. Femoral heads contoured up to lesser trochanter were not included in the PBM. The inner cavity of pelvic bone has been demonstrated by Mahantshetty U et al., to be a better surrogate of active bone marrow than whole pelvic bone so we delineated the inner cavity of all bones included in PBM [26]. PBM included the bone marrow of lumbar vertebrae (intervertebral disc were excluded) and sacrum (sacral foramina were excluded).
Treatment Planning
CT scan of all patients was performed in Siemens (Siemens Medical Solutions, Forchheim, Germany) 16 slice CT scanner using 2.0 mm slice thickness. As per the institution protocol, planning CT scans were performed with full bladder and empty rectum. The contouring was performed in Monaco v 5.11 (Elekta, Crawley, UK) contouring station of Elekta Synergy linear accelerator with a triple photon and seven electron energies. All 10 patient’s treatment plans were generated using Volumetric Arc Radiotherapy (VMAT) in Monaco (Version 5.11) treatment planning system using Monte Carlo Algorithm using two full arcs. Grid space for all plans was kept 3 mm along with minimum segment width of 5 mm. The planning optimizations were performed keeping all constraints in mind without compromising coverage of PTV.
The prescribed dose to PTV was 50 Gy in 25 fractions. The plans for BMS and Normal IMRT were generated in such a way that V95 of PTV was >95%, mean PTV dose was kept between 100-105% of prescribed dose. Constraints for bowel bag V35 was <35%, for rectum and bladder V40 was <40-60% and for both femoral head V35 was <10%. In addition to this, constraints to PBM were given in BMS-IMRT plans in such a way that V20 was <80% of prescribed dose [Table/Fig-2,3 and 4].
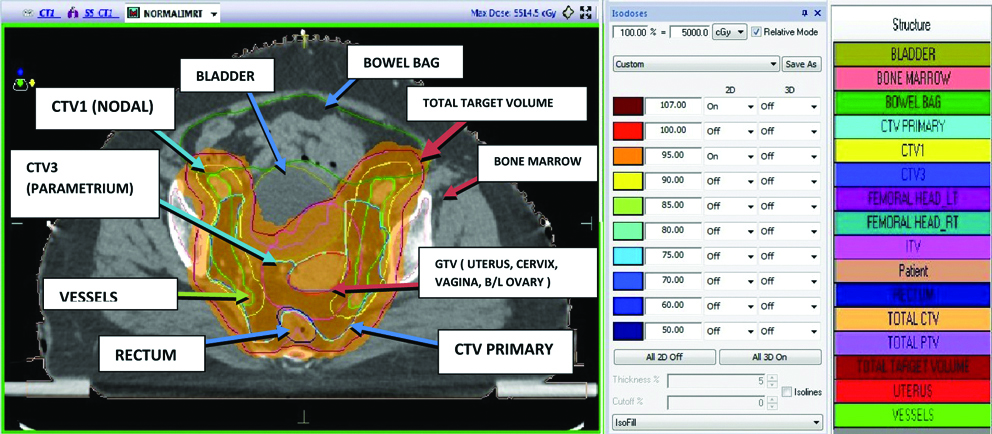
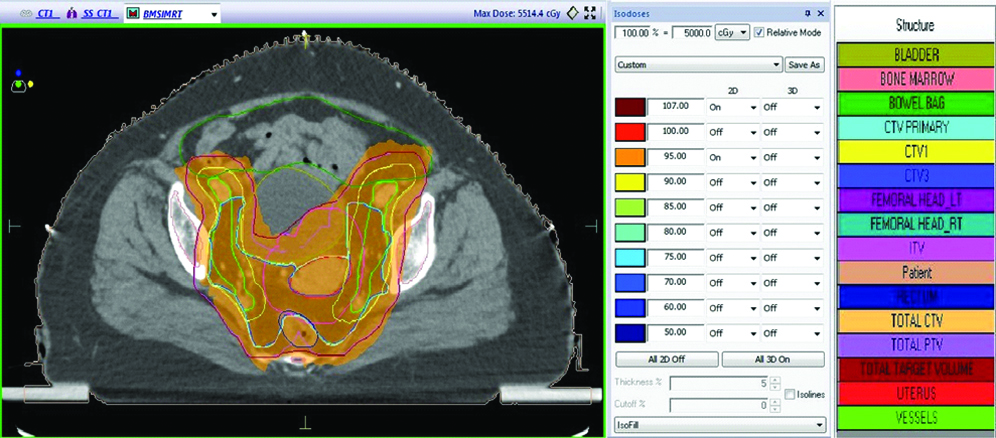
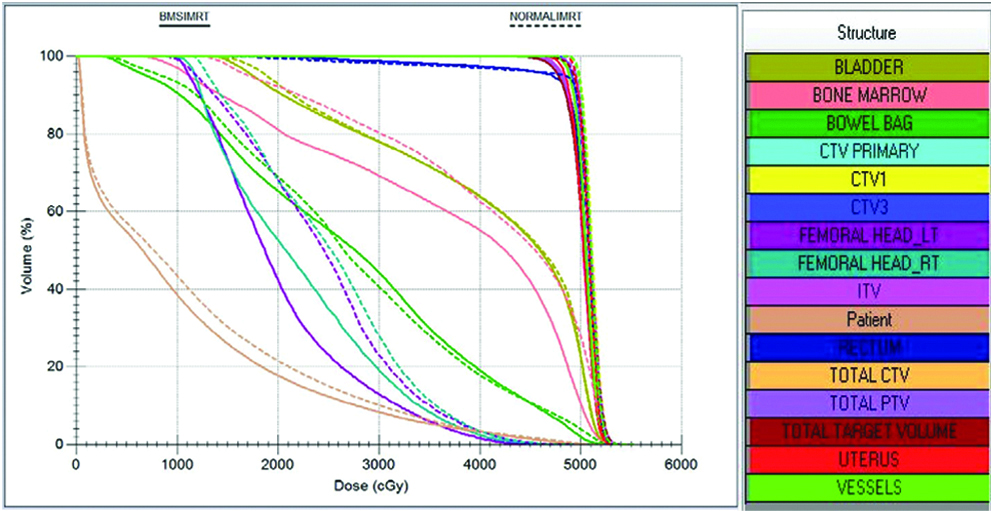
DVH showing normal and BMS-IMRT plan.
Statistical Analysis
An analysis was performed using SPSS 16.0 version. The mean values of all parameters in both the plans were compared with each other using Student’s paired t-test and the p-value of <0.05 was considered statistically significant.
Results
The mean values of PTV V95 was 99.02% and 97.68% (p-value= 0.016) and of V97 was 97.87% and 94.26% (p-value=0.019) for normal IMRT plans and BMS-IMRT plans respectively [Table/Fig-5]. PTV V95 was not compromised in any BMS-IMRT plan, however, mean values of PTV V95 was lower, and was statistically significant (p=0.016) in BMS-IMRT when compared to normal-IMRT.
PTV values (%) for Normal IMRT and BMS-IMRT plan.
| PTV | Parameters | Normal IMRT | BMS-IMRT |
|---|
| PTV Dmax | Mean | 109.718 | 109.894 |
| Median | 109.680 | 110.050 |
| PTV V95 | Mean | 99.026 | 97.689 |
| Median | 99.045 | 97.675 |
| PTV V97 | Mean | 97.870 | 94.268 |
| Median | 97.695 | 94.210 |
PTV: Planning target volume
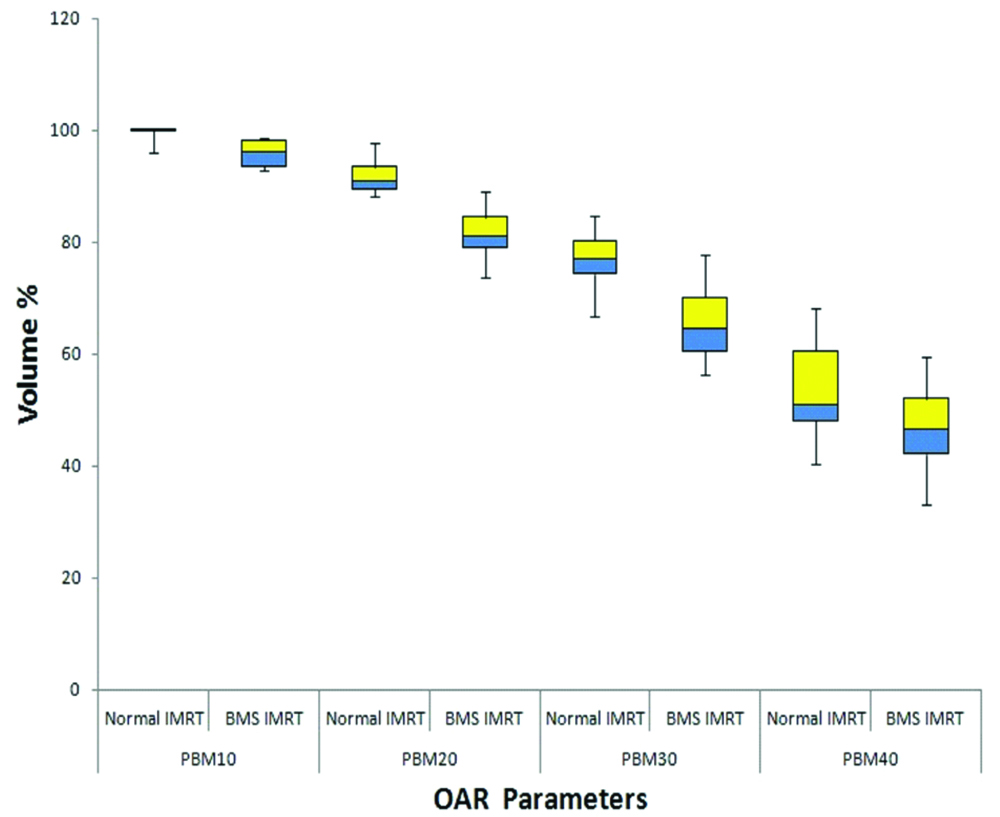
For PBM mean dose of V10, V20, V30, and V40 for normal IMRT and BMS-IMRT plans are shown in [Table/Fig-6,7]. When compared to normal-IMRT the mean values of PBM V10, V20, V30, V40 were lower and found to be statistically significant in BMS-IMRT.
Comparison of mean dose to the PBM.
| Parameter | Normal IMRT | BMS-IMRT | p-value |
|---|
| V10 | 99.50 | 96.01 | 0.01* |
| V20 | 92.09 | 81.66 | <0.001* |
| V30 | 77.12 | 66.72 | 0.002* |
| V40 | 53.48 | 46.89 | 0.016* |
*: Statistically significant
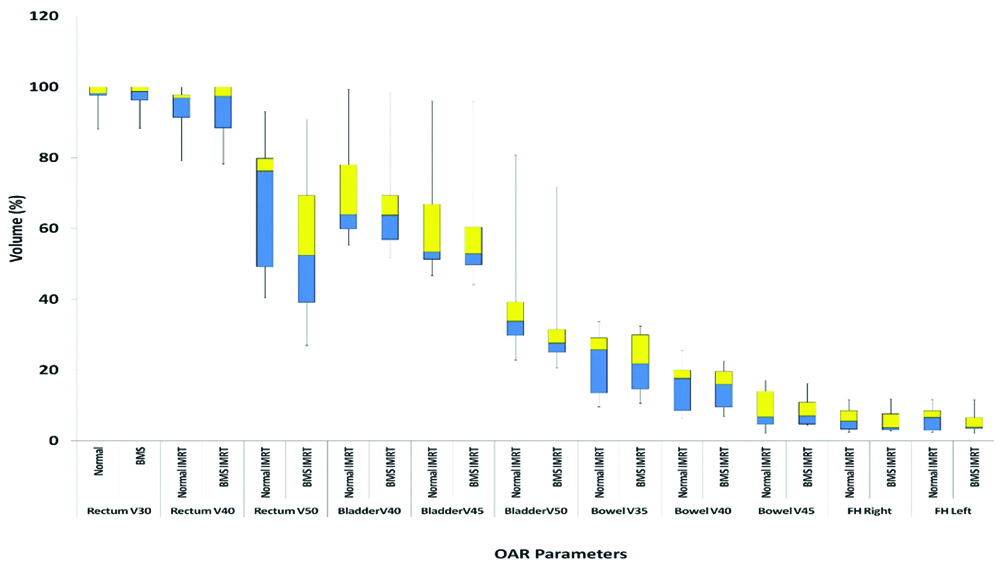
The mean values of rectum V30, V40 and V50, of bladder V40, V45, and V50, of bowel bag V35, V40, and V45, of femoral heads right and left, V35 for normal IMRT and BMS-IMRT are shown in [Table/Fig-8,9]. There was no statistically significant difference in the mean values of rectum V30, V40, bowel V35, V40, V45, and femoral head V35 between the two plans. However, the mean values of rectum V50, bladder V40, V45, and V50 were statistically lower in the case of BMS-IMRT.
Comparison of mean dose to the organs at risk.
| Parameter | Normal IMRT | BMS-IMRT | p-value |
|---|
| Bowel V35 | 22.50 | 22.70 | 0.76 |
| Bowel V40 | 15.15 | 15.28 | 0.86 |
| Bowel V45 | 9.14 | 8.97 | 0.71 |
| Rectum V30 | 97.14 | 97.08 | 0.78 |
| Rectum V40 | 93.5 | 93.23 | 0.5 |
| Rectum V50 | 68.34 | 58.02 | 0.01* |
| Bladder V40 | 70.25 | 66.49 | 0.01* |
| Bladder V45 | 61.56 | 58.47 | 0.008* |
| Bladder V50 | 40.38 | 30.44 | 0.01* |
| Femoral head right V35 | 6.21 | 5.61 | 0.22 |
| Femoral head left V35 | 6.21 | 3.38 | 0.28 |
*: Statistically significant
Discussion
Cervical cancer is the leading cancer in females in developing countries. The treatment for cervical cancer has evolved from conventional RT to conformal RT. This has led to a decrease in the bowel, rectal and bladder toxicity. Addition of chemotherapy concurrent with RT has further improved the survival rates [1-3], albeit at the cost of increase HT. PBM is the major site for haematopoiesis [27,28] and a majority of PBM is within the treatment field. Therefore, the HT toxicity is inevitable. The addition of chemotherapy to the RT further enhances the HT, with the consequence that many patients are not able to complete all 5 cycles of chemotherapy [3,29-31] or the treatment duration is prolonged because of interruption of treatment for ≥grade 2 HT with a negative impact on local control and overall survival [32-34]. Thus, there is a need to reduce the HT. Many studies have dosimetrically shown that BMS-IMRT reduces the dose to the PBM. From the various studies it has been shown that by keeping the PBM Dosimetric parameters of V10 to <90%, V20 to <80%, V30 to <65% and V40 to <37%, it is possible to reduce HT [5,7,35,36]. Rose BS et al., showed that if the volume of PBM receiving 10 Gy is >95% than the grade 3 neutropenia is more than in those patients in whom <95% of the PBM received this dose (63.8% vs 24.6%; p<0.001) [35]. In a study conducted by Albuquerque K reported that a volume of PBM receiving 20 Gy was most strongly predictive of grade ≥2 HT. When >80% of PBM received 20 Gy, the risk of HT increased by a factor of 4.5 [36]. In the present study V10, V20, V30, and V40 all were significantly lower in the BMS-IMRT plan with the maximum difference visible at V20. However, RTOG 0418 trial reported by Klopp AH et al., showed that if the volume of PBM receiving 40 Gy is >37% then the grade ≥ 2 HT is more than in the patients in whom V40 ≤37% (p=0.04) [7]. Although in our study the above constraints were not achieved, yet when compared to the normal IMRT plans, the mean values of all volumes were significantly less. The possible reasons which explain this could be that we delineated only the inner cavity of all included bones as bone marrow and we did not include bilateral femoral heads in the PBM which were given the constraint separately leading to a less absolute volume of PBM, whereas other studies have delineated the outer contour of bones as PBM. Other explanation could be that we conducted this dosimetric analysis on intact cervical patients wherein the PTV was considered generous. Also, all our patients received 50 Gy in 25 fractions where as most of the above studies gave 45 Gy in 25 fractions. Nonetheless sparing the PBM decreases the HT and increases patient’s compliance to the RT concurrent with chemotherapy. Many studies have shown that active bone marrow contoured on the basis of FDG PET is a better surrogate of HT [37-40]. Well-conducted randomized controlled trials and multi-institutional studies are required to establish whether this dosimetric gain will eventually be clinically effective or not.
One of the major caveat of this study was that the bilateral femoral heads were not included in the bone marrow, as according to the institutional protocol we give constraints to the femoral heads only, but for this retrospective Dosimetric study we contoured and gave the constraints to the PBM separately and so the femoral heads could not be included in the PBM. Another limitation of our study was that the constraints given to the bladder and rectum (V40≤40-60%) were not met. As the majority of rectum was lying within the total target volume, therefore its constraints could not be achieved. In case of bladder we were able to achieve, given constraints in 50% of our patients and in another half, it could not be achieved as most of the bladder was within the target volume. We realized that the constraints given to these organs were unrealistic. A study conducted by RTOG 0418 [41] in patients of endometrial carcinoma found that the constraints given to bladder and rectum of V45 ≤35% and V45 ≤60% could be achieved in only 33.3% and 22.8% respectively of their patients and they concluded that in all new trials loosening of dose constraints is recommended. In another dosimetric study by Mell LK et al., which compared BMS-IMRT with conventional radiotherapy techniques in seven intact cervical cancer patients, of which one patient was IB2 while six were locally advanced, V40 was 73.6% and 83.7% for bladder and rectum respectively [42]. The total dose given to the target volume was 45 Gy in 25 fractions. In the present study in 50% of our patients, bladder constraint could be achieved while in none of the patient’s rectal constraint was met. However, in comparison to normal IMRT plans the mean values of rectum V50, bladder V40, V45, and V50 were significantly lower in the case of BMS-IMRT.
Uterus-11 a prospective randomized German multicenter trial conducted by Marnitz S et al., evaluated the impact ofsurgical staging in FIGO IIB-IVA cervical cancer patients prior to Chemo-Radiation (CRT) [43]. This study reported that patients who underwent surgical staging prior to CRT experienced more ≥ grade 2 HT than the patients who primarily received CRT possibly because there was blood loss during surgery and more patients were treated with EFRT and hence more bone marrow volume inclusion within the field. They reported that the use of IMRT technique resulted in to more HT because of higher bone marrow volume covered by low dose as bone marrow was not spared. This study concluded that BMS-IMRT can reduce ≥grade 2 HT. Recently a study demonstrated the relationship between bone marrow response to radiation dose by using 18F-labeled fluorodeoxyglucose positron emission tomography Standard Uptake Values (SUV) and correlated these findings with HT in cervical cancer patients treated with CRT [40]. In this trial pelvic bone and lumbar spine were auto segmented as well as active bone marrow was delineated based on the mean SUV value. It showed that the volumes of total bone marrow and active bone marrow that are exposed to even relatively low doses of radiation were associated with a decrease in white blood cell counts following CRT. The loss in proliferative bone marrow SUV uptake translates into low WBC nadirs after treatment. This study concluded that the use of IMRT can spare total bone marrow and reduce long-term HT. In future, delineation of active bone marrow, using (18F) FDG-PET CT or MRI can be conducted and BMS-IMRT can be evaluated in prospective randomised controlled trials.
Conclusion
The need for intensified treatment for cervical cancer FIGO stage IB2-IVA is reflected in the poor survival rates but the aggressive approach is restricted because of toxicity of the surrounding normal structures and HT. Bone marrow sparing seems to be an effective way of reducing HT and thereby allowing completion of treatment with prescribed dose and concurrent chemotherapy in stipulated time. Further prospective randomised control trials are needed to establish this.
PTV: Planning target volume
*: Statistically significant
*: Statistically significant
[1]. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinomaN Engl J Med 1999 340(15):1154-61.10.1056/NEJM19990415340150310202166 [Google Scholar] [CrossRef] [PubMed]
[2]. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancerN Engl J Med 1999 340(15):1137-43.10.1056/NEJM19990415340150110202164 [Google Scholar] [CrossRef] [PubMed]
[3]. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancerN Engl J Med 1999 340(15):1144-53.10.1056/NEJM19990415340150210202165 [Google Scholar] [CrossRef] [PubMed]
[4]. Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysisThe Lancet 2001 358(9284):781-86.10.1016/S0140-6736(01)05965-7 [Google Scholar] [CrossRef]
[5]. Mell LK, Kochanski JD, Roeske JC, Haslam JJ, Mehta N, Yamada SD, Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapyInternational Int J Radiat Oncol Biol Phys 2006 66(5):1356-65.10.1016/j.ijrobp.2006.03.01816757127 [Google Scholar] [CrossRef] [PubMed]
[6]. Mell LK, Schomas DA, Salama JK, Devisetty K, Aydogan B, Miller RC, Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapyInt J Radiat Oncol Biol Phys 2008 70(5):1431-37.10.1016/j.ijrobp.2007.08.07417996390 [Google Scholar] [CrossRef] [PubMed]
[7]. Klopp AH, Moughan J, Portelance L, Miller BE, Salehpour MR, Hildebrandt E, Hematologic toxicity in RTOG 0418: A phase 2 study of postoperative IMRT for gynecologic cancerInt J Radiat Oncol Biol Phys 2013 86(1):83-90.10.1016/j.ijrobp.2013.01.01723582248 [Google Scholar] [CrossRef] [PubMed]
[8]. Toledano I, Graff P, Serre A, Boisselier P, Bensadoun RJ, Ortholan C, Intensity-modulated radiotherapy in head and neck cancer: Results of the prospective study GORTEC 2004-03Radiotherapy and Oncology 2012 103(1):57-62.10.1016/j.radonc.2011.12.01022296746 [Google Scholar] [CrossRef] [PubMed]
[9]. Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomized controlled trialThe Lancet Oncology 2011 12(2):127-36.10.1016/S1470-2045(10)70290-4 [Google Scholar] [CrossRef]
[10]. Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X, Improved clinical outcomes with high-dose image-guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancerInt J Radiat Oncol Biol Phys 2012 84(1):125-29.10.1016/j.ijrobp.2011.11.04722330997 [Google Scholar] [CrossRef] [PubMed]
[11]. Alicikus ZA, Yamada Y, Zhang Z, Pei X, Hunt M, Kollmeier M, Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancerCancer 2011 117(7):1429-37.10.1002/cncr.2546721425143 [Google Scholar] [CrossRef] [PubMed]
[12]. Roeske JC, Lujan A, Rotmensch J, Waggoner SE, Yamada D, Mundt AJ, Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignanciesInt J Radiat Oncol Biol Phys 2000 48(5):1613-21.10.1016/S0360-3016(00)00771-9 [Google Scholar] [CrossRef]
[13]. Brixey CJ, Roeske JC, Lujan AE, Yamada SD, Rotmensch J, Mundt AJ, Impact of intensity modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignanciesInt J Radiat Oncol Biol Phys 2002 54(5):1388-96.10.1016/S0360-3016(02)03801-4 [Google Scholar] [CrossRef]
[14]. Mundt AJ, Lujan AE, Rotmensch J, Waggoner SE, Yamada SD, Fleming G, Intensity modulated whole pelvic radiotherapy in women with gynecologic malignanciesInt J Radiat Oncol Biol Phys 2002 52(5):1330-37.10.1016/S0360-3016(01)02785-7 [Google Scholar] [CrossRef]
[15]. Lujan AE, Mundt AJ, Yamada SD, Rotmensch J, Roeske JC, Intensity-modulated radiotherapy as a means of reducing dose to bone marrow in gynecologic patients receiving whole pelvic radiotherapyInt J Radiat Oncol Biol Phys 2003 57(2):516-21.10.1016/S0360-3016(03)00521-2 [Google Scholar] [CrossRef]
[16]. Murakami N, Okamoto H, Kasamatsu T, Kobayashi K, Harada K, Kitaguchi M, A dosimetric analysis of intensity-modulated radiation therapy with bone marrow sparing for cervical cancerAnticancer Research 2014 34(9):5091-98. [Google Scholar]
[17]. FIGO Committee on Gynecologic OncologyFIGO staging for carcinoma of the vulva, cervix, and corpus uteriInt J Gynaecol Obstet 2014 125(2):97-98.10.1016/j.ijgo.2014.02.00324630859 [Google Scholar] [CrossRef] [PubMed]
[18]. Taylor A, Rockall AG, Reznek RH, Powell ME, Mapping pelvic lymph nodes: guidelines for delineation in intensity-modulated radiotherapyInt J Radiat Oncol Biol Phys 2005 63(5):1604-12.10.1016/j.ijrobp.2005.05.06216198509 [Google Scholar] [CrossRef] [PubMed]
[19]. Taylor A, Rockall AG, Powell ME, An atlas of the pelvic lymph node regions to aid radiotherapy target volume definitionClinical Oncology 2007 19(7):542-50.10.1016/j.clon.2007.05.00217624745 [Google Scholar] [CrossRef] [PubMed]
[20]. Small W, Mell LK, Anderson P, Creutzberg C, De Los Santos J, Gaffney D, Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancerInt J Radiat Oncol Biol Phys 2008 71(2):428-34.10.1016/j.ijrobp.2007.09.04218037584 [Google Scholar] [CrossRef] [PubMed]
[21]. Toita T, Ohno T, Kaneyasu Y, Uno T, Yoshimura R, Kodaira T, A consensus-based guideline defining the clinical target volume for pelvic lymph nodes in external beam radiotherapy for uterine cervical cancerJpn J Clin Oncol 2010 40(5):456-63.10.1093/jjco/hyp19120133334 [Google Scholar] [CrossRef] [PubMed]
[22]. Toita T, Ohno T, Kaneyasu Y, Kato T, Uno T, Hatano K, A consensus-based guideline defining clinical target volume for primary disease in external beam radiotherapy for intact uterine cervical cancerJpn J Clin Oncol 2011 41(9):1119-26.10.1093/jjco/hyr09621875938 [Google Scholar] [CrossRef] [PubMed]
[23]. Lim K, Small W, Portelance L, Creutzberg C, Jürgenliemk-Schulz IM, Mundt A, onsensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancerInt J Radiat Oncol Biol Phys 2011 79(2):348-55.10.1016/j.ijrobp.2009.10.07520472347 [Google Scholar] [CrossRef] [PubMed]
[24]. Bansal A, Patel FD, Rai B, Gulia A, Dhanireddy B, Sharma SC, A literature review with PGI guidelines for delineation of clinical target volume for intact carcinoma cervixJ Can Res Ther 2013 9(4):57410.4103/0973-1482.12645024518699 [Google Scholar] [CrossRef] [PubMed]
[25]. Gay HA, Barthold HJ, O’Meara E, Bosch WR, El Naqa I, Al-Lozi R, Pelvic normal tissue contouring guidelines for radiation therapy: A Radiation Therapy Oncology Group consensus panel atlasInt J Radiat Oncol Biol Phys 2012 83(3):e353-62.10.1016/j.ijrobp.2012.01.02322483697 [Google Scholar] [CrossRef] [PubMed]
[26]. Mahantshetty U, Krishnatry R, Chaudhari S, Kanaujia A, Engineer R, Chopra S, Comparison of 2 contouring methods of bone marrow on CT and Correlation with hematological toxicities in non-bone marrow-sparing pelvic intensity-modulated radiotherapy with concurrent cisplatin for cervical cancerInt J Gynecol Cancer 2012 22(8):1427-34.10.1097/IGC.0b013e3182664b4622932264 [Google Scholar] [CrossRef] [PubMed]
[27]. Ellis RE, The distribution of active bone marrow in the adultPhysics in Medicine & Biology 1961 5(3):25510.1088/0031-9155/5/3/30213726497 [Google Scholar] [CrossRef] [PubMed]
[28]. Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, Hematopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapyInt J Radiat Oncol Biol Phys 1995 31(5):1319-39.10.1016/0360-3016(94)00430-S [Google Scholar] [CrossRef]
[29]. Peters III WA, Liu PY, Barrett RJ, Stock RJ, Monk BJ, Berek JS, Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervixJ Clin Oncol 2000 Apr 18(8):1606-13.10.1200/JCO.2000.18.8.160610764420 [Google Scholar] [CrossRef] [PubMed]
[30]. Torres MA, Jhingran A, Thames Jr HD, Levenback CF, Bodurka DC, Ramondetta LM, Comparison of treatment tolerance and outcomes in patients with cervical cancer treated with concurrent chemoradiotherapy in a prospective randomized trial or with standard treatmentInt J Radiat Oncol Biol Phys 2008 70(1):118-25.10.1016/j.ijrobp.2007.05.02817869451 [Google Scholar] [CrossRef] [PubMed]
[31]. Abu-Rustum NR, Lee S, Correa A, Massad LS, Compliance with and acute hematologic toxic effects of chemoradiation in indigent women with cervical cancerGynecologic Oncology 2001 81(1):88-91.10.1006/gyno.2000.610911277656 [Google Scholar] [CrossRef] [PubMed]
[32]. Parker K, Gallop-Evans E, Hanna L, Adams M, Five years’ experience treating locally advanced cervical cancer with concurrent chemoradiotherapy and high-dose-rate brachytherapy: results from a single institutionInt J Radiat Oncol Biol Phys 2009 74(1):140-46.10.1016/j.ijrobp.2008.06.192018922646 [Google Scholar] [CrossRef] [PubMed]
[33]. Lanciano RM, Pajak TF, Martz K, Hanks GE, The influence of treatment time on an outcome for squamous cell cancer of the uterine cervix treated with radiation: A patterns-of-care studyInt J Radiat Oncol Biol Phys 1993 25(3):391-97.10.1016/0360-3016(93)90058-4 [Google Scholar] [CrossRef]
[34]. Perez CA, Grigsby PW, Castro-Vita H, Lockett MA, Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on the outcome of radiation therapyInt J Radiat Oncol Biol Phys 1995 32(5):1275-88.10.1016/0360-3016(95)00220-S [Google Scholar] [CrossRef]
[35]. Rose BS, Aydogan B, Liang Y, Yeginer M, Hasselle MD, Dandekar V, Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapyInt J Radiat Oncol Biol Phys 2011 79(3):800-07.10.1016/j.ijrobp.2009.11.01020400238 [Google Scholar] [CrossRef] [PubMed]
[36]. Albuquerque K, Giangreco D, Morrison C, Siddiqui M, Sinacore J, Potkul R, Radiation related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow-sparing pelvic IMRTInt J Radiat Oncol Biol Phys 2011 79(4):1043-47.10.1016/j.ijrobp.2009.12.02520471182 [Google Scholar] [CrossRef] [PubMed]
[37]. Rose BS, Liang Y, Lau SK, Jensen LG, Yashar CM, Hoh CK, Correlation between radiation dose to 18F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapyInt J Radiat Oncol Biol Phys 2012 83(4):1185-91.10.1016/j.ijrobp.2011.09.04822270171 [Google Scholar] [CrossRef] [PubMed]
[38]. Liang Y, Bydder M, Yashar CM, Rose BS, Cornell M, Hoh CK, A prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic malignanciesInt J Radiat Oncol Biol Phys 2013 85(2):406-14.10.1016/j.ijrobp.2012.04.04422687195 [Google Scholar] [CrossRef] [PubMed]
[39]. McGuire SM, Menda Y, Ponto LL, Gross B, Juweid M, Bayouth JE, A methodology for incorporating functional bone marrow sparing in IMRT planning for pelvic radiation therapyRadiotherapy and Oncology 2011 99(1):49-54.10.1016/j.radonc.2011.01.02521397965 [Google Scholar] [CrossRef] [PubMed]
[40]. Elicin O, Callaway S, Prior JO, Bourhis J, Ozsahin M, Herrera FG, [18F] FDG-PET standard uptake value as a metabolic predictor of bone marrow response to radiation: impact on acute and late hematological toxicity in cervical cancer patients treated with chemoradiation therapyInt J Radiat Oncol Biol Phys 2014 90(5):1099-107.10.1016/j.ijrobp.2014.08.01725442041 [Google Scholar] [CrossRef] [PubMed]
[41]. Jhingran A, Winter K, Portelance L, Miller B, Salehpour M, Gaur R, A phase II study of intensity modulated radiation therapy to the pelvis for postoperative patients with endometrial carcinoma: radiation therapy oncology group trial 0418Int J Radiat Oncol Biol Phys 2012 84(1):e23-28.10.1016/j.ijrobp.2012.02.04422543211 [Google Scholar] [CrossRef] [PubMed]
[42]. Mell LK, Tiryaki H, Ahn KH, Mundt AJ, Roeske JC, Aydogan B, Dosimetric comparison of bone marrow-sparing intensity-modulated radiotherapy versus conventional techniques for treatment of cervical cancerInt J Radiat Oncol Biol Phys 2008 71(5):1504-10.10.1016/j.ijrobp.2008.04.04618640499 [Google Scholar] [CrossRef] [PubMed]
[43]. Marnitz S, Martus P, Köhler C, Stromberger C, Asse E, Mallmann P, Role of surgical versus clinical staging in chemoradiated FIGO stage IIB-IVA cervical cancer patients-acute toxicity and treatment quality of the uterus-11 multicenter phase III Intergroup Trial of the German Radiation Oncology Group and the Gynecologic Cancer GroupInt J Radiat Oncol Biol Phys 2016 94(2):243-53.10.1016/j.ijrobp.2015.10.02726853333 [Google Scholar] [CrossRef] [PubMed]