WP-CRT is still the most widely practiced method of External Beam Radiotherapy (EBRT) in the definitive management of Locally Advanced Carcinoma of Cervix (LACC). WP-CRT with Concurrent Chemotherapy (CCRT) leads to significant acute Grade-3-4 non-haematological (20-30%) and haematological toxicities (30-40%) [1]. Limited studies on long term outcome suggest a 10-20% rate of significant late toxicities [2].
Dosimetric studies have shown that WP-IMRT reduces dose to rectum, bladder, small bowel, pelvic bone marrow [3–7]. Clinical studies, though mostly non-randomized, have confirmed the translation of this dosimetric advantage into significant reduction of acute Gastrointestinal (GI), Genitourinary (GU) and haematological toxicities [8–13]. Chronic GI toxicities have also been found to be significantly less with WP-IMRT as compared to WP-CRT [9,14]; however, the data on chronic GU toxicities remain limited.
Materials and Methods
Patient Selection
This was a non-blinded, prospective, parallel randomised trial. Between January 2010 and January 2012, 44 patients were recruited at Department of Radiation Oncology, All India Institute of Medical Sciences, New Delhi, India. Patients of uterine cervix {International Federation of Gynaecology and Obstetrics (FIGO 2009) stage IIB-IIIB} with squamous cell carcinoma were eligible for this study. Patients were of age between 25-65 years, Karnofsky Performance Status (KPS) ≥70, complete blood count, kidney function test results and liver function test results within normal limits. Patients with gross lymph node positivity were excluded from the study. The study had ethical clearance from the institution (IEC Number: 23/Res Cell/AIIMS/2010) and all participating subjects gave their informed written consent prior to accrual in to the study.
Study Design
Patients were randomized to either WP-IMRT or WP-CRT arms by computer aided random numbers. EBRT dose was 50.4 Gray in 28 fractions over 5.5 weeks in both the treatment arms. Cisplatin 40 mg/m2 intravenous weekly during the course of CCRT was given in both the arms. Brachytherapy was delivered after completion of EBRT and either High Dose Rate (HDR) Intracavitary Brachytherapy (ICBT), 7 Gray to point A (3 sessions, each one week apart) or in patients deemed unfit for ICBT, 10 Gray (2 sessions, each one week apart) of Interstitial Brachytherapy (ISBT) was used.
Treatment at Recurrence
At the time of failure, patients were evaluated for salvage treatment based on the site and extent of recurrence, disease free interval, performance status and willingness of the patient. Patients with isolated local failures were evaluated for either surgery or reirradiation. Reirradiation was done with ISBT with or without further EBRT. Patients with distant metastasis (isolated or synchronous with local failure) were treated with palliative intent chemotherapy with paclitaxel (175 mg/m2 intravenous three weekly; day 1) and carboplatin (AUC 5-6 intravenous three weekly; day 2). Patients with isolated local failures not suitable for either surgery or reirradiation were also treated with chemotherapy. Palliative radiotherapy to symptomatic sites were used, 20 Gray in 5 fractions over 1 week for lung and brain metastasis and 8 Gray in single fraction for bone metastasis.
Follow-Up and Evaluation of Toxicity
Response to salvage treatment was assessed with WHO response assessment criteria 1981 [16]. All patients were followed up monthly for first six months, thereafter every three months till two years, and afterwards six monthly till date. At each follow-up, apart from a complete physical and gynecological examination, complete blood counts and blood chemistry tests were ordered. Contrast Enhanced Computed Tomography (CECT) scan of the abdomen and pelvis or whole-body Positron Emission Tomography-CT (PET-CT) was done at six month follow-up and then at every six months interval or earlier based on clinical suspicion till five years. After five years, imaging was done only based on clinical suspicion. Late toxicities were defined after 90 days of completion of treatment and were Graded as per morbidity scoring criteria by Radiation Therapy Oncology Group (RTOG) [17].
Statistical Analysis
Survival outcomes were measured from the date of initiation of CCRT. Loco-Regional Failure Free Survival (LFFS) was defined from initiation date till the occurrence of a pelvic or regional lymph node recurrence. Distant Metastasis Free Survival (DMFS) was measured from initiation date till occurrence of a distant metastasis. Disease Free Survival (DFS) was measured from the initiation to the first event of loco-regional failure or distant metastasis. Patients without any event were censored at the time of the last follow-up or death. Overall survival was defined from the initiation date to time of death from any cause. Sample size was not calculated for the primary endpoint and was limited to 44 on the basis of available resources. Survival outcomes were estimated by Kaplan-Meier method. χ2test/Fisher’s-exact test was used to compare toxicity between arms. SPSS Software, version 21.0 (SPSS Inc, Chicago, IL), was used for all data analyses, all p-values were based on a 2-sided hypothesis and p-value <0.05 were considered significant.
Results
Of 44 patients, 22 patients each received WP-CRT and WP-IMRT respectively. Median age of patients in the WP-CRT and WP-IMRT arms were 45 years (35-65) and 50 years (35-65) respectively. Of 44 patients, 13 and 12 patients belonged to stage IIB; 9 and 10 patients belonged to stage IIIB in WP-CRT and WP-IMRT arm respectively. Median KPS of patients in both the arms were 90 (70-90).
Toxicity
Dose volume histogram characteristics for organ at risks have been summarized in [Table/Fig-1]. Comparative toxicities have been documented in [Table/Fig-1]. Overall for combined Grade-1-3, patients in the WP-IMRT arm experienced significantly fewer chronic GI toxicity (18.2% vs. 50%, p=0.027). In the WP-IMRT arm, the percentage of patients having Grade-1, 2 and 3 chronic GI toxicities were 13.6%,4.5% and 0% respectively while for patients in the conventional arm, the rates were 27.3%, 13.6% and 9.1%. Grade ≥2 and Grade ≥3 chronic toxicity was observed in 18.8% vs 9.09% (p=0.664) and 13.63% vs 0% (p=0.116), respectively, in the WP-CRT and WP-IMRT arms. Most Grade-1 GI toxicities were in the form of increased frequency, indigestion or urgency. Grade-2 GI toxicities consisted of intermittent bleeding in 2 patients and excessive rectal mucus discharge along with intermittent bleeding in 1 patient and increased colic movement and diarrhea in 1 patient. Grade-3 GI toxicity in two patients (both in conventional arm) were in the form of severe proctitis and bleeding requiring transfusion in one and obstruction requiring surgery in another. Mean V90 and V100 of SB in CRT and IMRT arm was 417.54 vs 199.89 mL and 102.47 vs 336.22 mL, respectively. V90, V100 and V45 > 180 cc of SB correlated with acute GI toxicity (p=0.04, p=0.031, and p=0.036) but not with chronic GI toxicity. Volume of the rectum did not correlate significantly with either acute or chronic gastrointestinal toxicity (all p>0.05).
Comparative toxicity and dose volume histogram characteristics for organs at risk.
| WP-CRT arm | WP-IMRT arm | p-value |
|---|
| GI Grade ≥1 (% of patients) | 50% | 18.2% | 0.027 |
| GI Grade ≥ 2 (% of patients) | 18.8% | 9.1% | 0.664 |
| GI Grade ≥ 3 (% of patients) | 13.6% | 0% | 0.116 |
| GU Grade ≥ 2 (% of patients) | 13.6% | 0% | 0.116 |
| Mean rectum V40 (% volume) | 98.37±4.58 | 42±2.78 | 0.0001 |
| Mean bladder V40 (% volume) | 97.54±3.78 | 42.44±2.74 | 0.0001 |
| Mean small bowel V40 (% volume) | 61.21±14.63 | 31.66±3.56 | 0.001 |
| Mean small bowel V90 (Volume in cm3) | 417.54±42.16 | 199.89±47.08 | 0.005 |
GI: Gastrointestinal; GU: Genitourinary; WP-CRT: Whole pelvic conventional radiotherapy; WP-IMRT: Whole pelvic intensity modulated radiotherapy. Planning constraints for normal tissues were as follows: 1) Small bowel: volume receiving 40 Gy (V40) < 32%; maximum dose < 50 Gy; 2) Rectum: V40 < 40%; maximum dose < 50 Gy; 3) Bladder: V40 < 40%; maximum dose < 50 Gy.
One patient in each arm developed chronic Grade-1 bladder toxicity. Both of these patients had increased frequency of urination. WP-CRT arm showed a trend towards more Grade-2 late bladder toxicity as compared to WP-IMRT arm (13.6% vs. 0%, p=0.116). Of the three patients in conventional arm who developed Grade-2 bladder toxicity, two patients had severe dysuria and increased frequency and 1 patient had macroscopic haematuria.
Patterns of Failure and Salvage Treatment
The median follow-up time for patients in the WP-IMRT was 46.7 months (range, 22.8-59.8 months) and for the WP-CRT arm was 51.23 months (range, 21.7-59.8 months). At the time of last follow-up visit, there were two isolated local failures, one each in both the arms. Seven patients had only distant metastases, four in the WP-CRT arm and three in the WP-IMRT arm. Three patients had failure both at the local as well as distant site, two in WP-IMRT arm and one in WP-CRT arm. The median time to local failure was 11.2 months (range, 7.4-11.5 months) and the median time to distant metastases was 21.5 months (range, 6.3-53.2 months). The different sites of local and distant failures, details of the salvage treatment and responses have been summarized in [Table/Fig-2].
Patterns of failure and details of salvage treatment of the patients.
| S no. | Arm | Local recurrence | Sites of distant metastasis | Salvage treatment received | Mode of diagnosis | Response to salvage treatment |
|---|
| 1 | WP-CRT | No | Para-Aortic | Para-aortic irradiation f/b Palliative CT | PET-CT | PR |
| 2 | WP-CRT | Yes | Lung metastasis | ISBT 15 Gray in 5 fractions Palliative CT | Clinical examination and CECT | PR at both local and distant sites |
| 3 | WP-CRT | Yes | No | ISBT 15 Gray in 5 fractions Palliative CT | Clinical examination | PD |
| 4 | WP-CRT | No | Brain, lung, liver, bone metastasis | Palliative RT (Brain and Bone) BSC | CECT | PD |
| 5 | WP-CRT | No | Lung metastasis | Palliative CT | CECT | PR |
| 6 | WP-CRT | No | Endo-bronchial metastasis f/b Brain metastasis | Palliative CT Palliative RT (Brain) | CECT | PR |
| 7 | WP-IMRT | No | Lung metastasis | Palliative CT | CECT | PR |
| 8 | WP-IMRT | Yes | No | Palliative CT | Clinical examination/ PET-CT | PD |
| 9 | WP-IMRT | Yes | Para-Aortic, Adrenal, Liver, Lung metastasis | Palliative CT | Clinical examination/ PET-CT | PR |
| 10 | WP-IMRT | No | Multiple liver metastasis | Palliative CT | CECT | PD |
| 11 | WP-IMRT | No | Isolated inguinal recurrence f/b Supraclavicular and lung metastasis | Inguinal dissection with PORT and later palliative CT | PET-CT | CR to initial treatment f/b PR |
| 12 | WP-IMRT | Yes | Lung metastasis | Palliative RT (Lung) Palliative CT | Clinical examination/ PET-CT | PR |
WP-CRT: Whole pelvic conventional radiotherapy; WP-IMRT: Whole pelvic intensity modulated radiotherapy; CT: Chemotherapy; ISBT: Interstitial brachytherapy; RT: Radiotherapy; BSC: Best supportive care; PR: Partial response; CR: Complete response; PD: Progressive disease; PORT: Post-operative radiotherapy; f/b: Followed by; PET-CT: 18F-Fluoro-deoxy glucose positron emission tomography-computed tomography; CECT: Contrast enhanced computed tomography
Eleven patients received palliative chemotherapy at some point of time after recurrence and 2 patients received local reirradiation with ISBT. Para-aortic irradiation to a prescription dose of 45 Gray in 25 fractions over five weeks plus boost of 10 Gray in 5 fractions over one week was used in one patient with isolated para-aortic failure (Patient number 1, [Table/Fig-2]). However, this patient later progressed and received palliative chemotherapy. Three patients received palliative radiotherapy, 1 to brain, another to lung and 3rd to both brain and bone. One patient (patient number 11, [Table/Fig-2]) developing isolated inguinal recurrence underwent inguinal dissection followed by post-operative radiotherapy to tumour bed to a prescription dose of 45 Gray in 25 fractions over five weeks. However, she later developed further distant metastasis and received palliative chemotherapy. Initial response to salvage treatment was complete response in one patient, partial response in seven patients and progressive disease in four patients.
Survival Outcomes
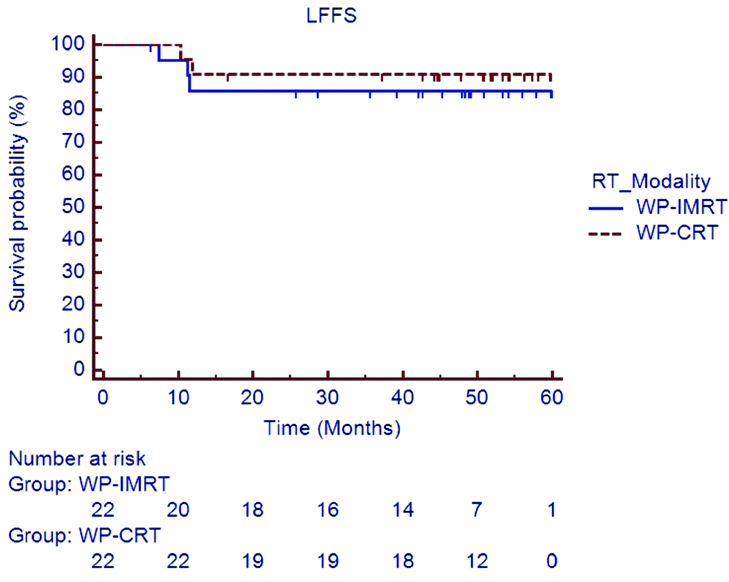
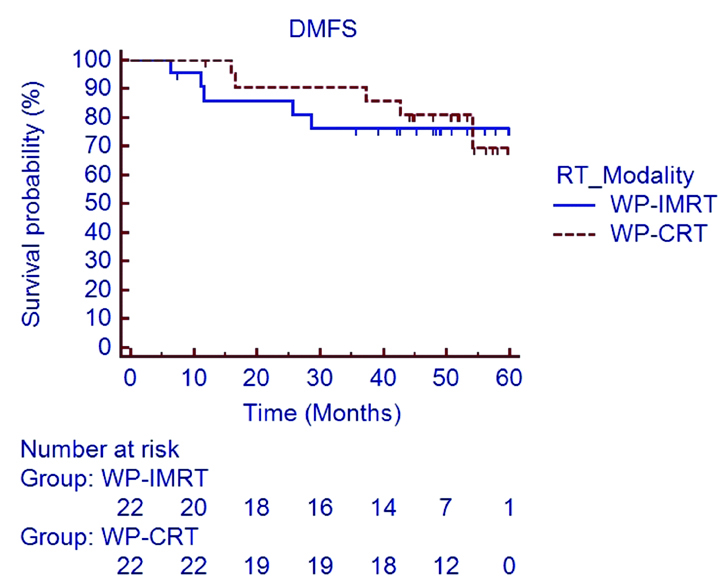
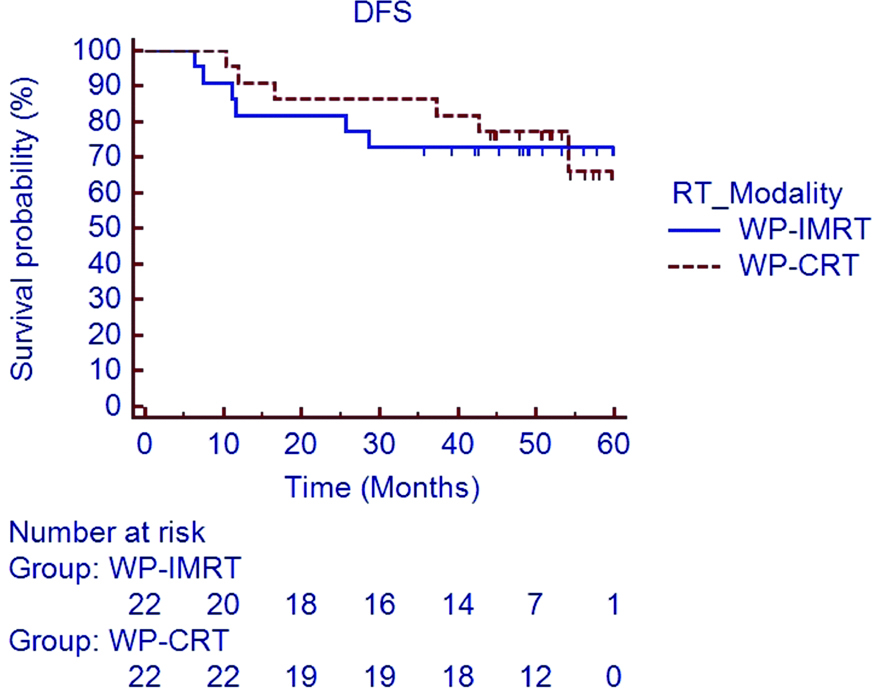
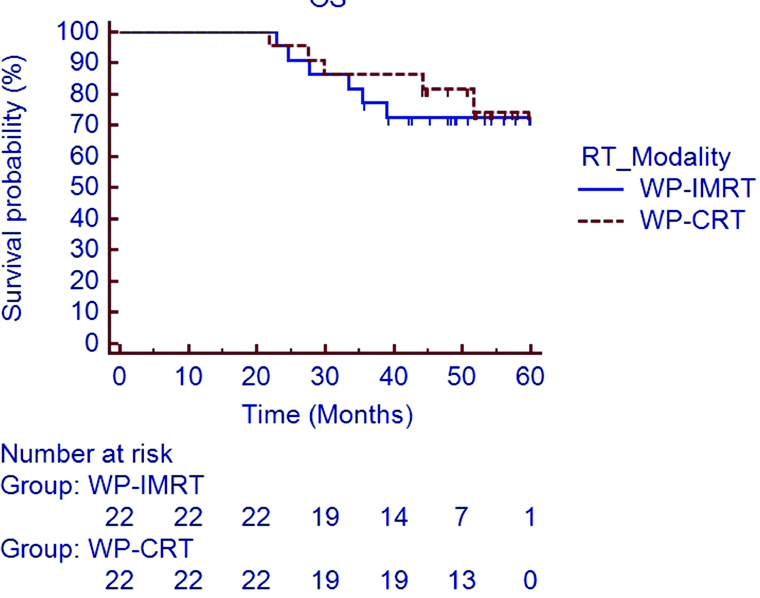
At the time of last follow-up visit, 11 patients had died (five patients in WP-CRT arm and six patients in WP-IMRT arm). Estimated 5 year LFFS [Table/Fig-3] and DMFS [Table/Fig-4] rates in the WP-IMRT arm versus the WP-CRT arm were 85.7% versus 90.9% (HR 0.609; 95% CI 0.105 to 3.527; p=0.58) and 76.4% versus 69.4% (HR 0.849; 95% CI 0.245 to 2.94; p=0.79). The 5 year estimated DFS [Table/Fig-5] and OS [Table/Fig-6] rate in the WP-IMRT arm versus the WP-CRT were 72.7% versus 66.2% (HR 0.869; 95% CI 0.279 to 2.7; p=0.80) and 72.4% versus 74.4% (HR 0.739; 95% CI 0.226 to 2.415; p=0.61).
Loco-regional failure free survival in WP-IMRT and WP-CRT arms.
LFFS: Loco-regional failure free survival; WP-IMRT: Whole pelvic intensity modulated radiotherapy; WP-CRT: Whole pelvic conventional radiotherapy
Distant metastasis free survival in WP-IMRT and WP-CRT arms.
DMFS: Distant metastasis free survival; WP-IMRT: Whole pelvic intensity modulated radiotherapy; WP-CRT: Whole pelvic conventional radiotherapy
Disease free survival in WP-IMRT and WP-CRT arms.
DFS: Disease free survival; WP-IMRT: Whole pelvic intensity modulated radiotherapy; WP-CRT: Whole pelvic conventional radiotherapy
Overall Survival in WP-IMRT and WP-CRT arms.
OS: Overall survival; WP-IMRT: Whole pelvic intensity modulated radiotherapy; WP-CRT: Whole pelvic conventional radiotherapy
Discussion
Whole pelvic radiotherapy with concurrent chemotherapy (platinum based) combined with ICBT or interstitial brachytherapy (in patients unsuitable for ICBT) is the current standard of management for LACC [18]. Five year DFS and OS with this approach has been reported at best to be around 50-60%. Loco-regional recurrence rates have also been found to be approximately 30-40% [18]. Pelvic control rates in the recent series with incorporation of magnetic resonance imaging-based image guided brachytherapy have improved to 85-90% [19]. This moderate outcome comes at the cost of significant haematological, GI and GU toxicities; both acute and chronic [1]. Trials addressing modification of the existing cisplatin based CCRT regimens in a zest to reduce toxicities have not yielded any positive outcomes [20-22]. Dueñas-González A et al., reported an improvement in OS (HR 0.68; 95% CI, 0.49 to 0.95; p=0.022) over the existing CCRT regimen [23]. The authors treated the patients in their randomized phase III trial with cisplatin (40 mg/m2 weekly) plus gemcitabine (125 mg/m2 weekly) CCRT followed by two adjuvant chemotherapy cycles (3 week apart) with cisplatin (50 mg/m2 on day 1) and gemcitabine (1000 mg/m2 on day 1 and 8) versus cisplatin CCRT. However, the enthusiasm of this survival advantage was offset by overwhelming Grade-3-4 toxicities of 86.5% versus 46.3% (p <0.001) in the control arm.
WP-IMRT has been shown to reduce acute as well as late toxicities [8–10,12–15] and this could be a great opportunity to combine the toxicity sparing effect of WP-IMRT with the intensified regimens such as that suggested by Dueñas-González A et al., [23]. Despite the increasing use of WP-IMRT, as well as encouraging accrual in multi-institutional prospective trial [24]; the routine use of WP-IMRT may be halted by the lack of data on long term efficacy and toxicity of WP-IMRT compared to the conventional counterpart.
In an update of our earlier published work [9], we noted in the present study that the 5-year LFFS (85.7% vs. 90.9%; p=0.58) and 5-year DMFS (76.4% vs. 69.4%; p=0.79) were not statistically different between WP-IMRT and WP-CRT respectively. The 5-year OS (72.4% vs. 74.4%; p=0.61) and 5-year DFS (72.7% vs. 66.2%; p=0.80) were also comparable between WP-IMRT and WP-CRT arms, respectively. The survival rate in both the arms, albeit a bit better, is also commensurate with the current reported outcomes for LACC treated with cisplatin based CCRT [18]. The median time to loco-regional failure for the entire cohort was 11.2 months (7.4 to 11.5 months) and for distant metastasis was 21.5 months (6.3 to 53.2 months). Since, most of the loco-regional failures in carcinoma cervix occur within 2-3 years of completion of treatment, it is unlikely that further follow-up beyond 5 years would change loco-regional control outcomes in our study. Based on this hypothesis, it seems reasonably safe to state that WP-IMRT is associated with comparable long term clinical outcome as compared to WP-CRT.
Late toxicities, often ignored and under-reported after CCRT usually tend to get worse over period of time. Eifel PJ et al., reported Grade-3 late toxicity of approximately 8% and 10% at 3 and 5 years of follow-up respectively [2]. The risk also increases on continued follow-up at roughly 0.34% per year [2]. Chronic GI toxicity (Grade-1-3) was found be significantly worse in WP-CRT as compared to WP-IMRT (50.2% vs. 18.2%; p=0.027) in our present study. The rates of Grade-3 chronic GI toxicity (9.1% vs. 0%) and Grade-2 bladder toxicity (13.6% vs. 0%) were also higher in WP-CRT arm, although statistically not significant. In keeping with the earlier discussion, since the rates of chronic toxicities are time dependent and often continue to increase beyond 10-20 years of follow-up; it would be premature to draw any conclusion at the present follow-up of 5 years. However, lower rates of significant toxicities (Grade-2 or higher) in WP-IMRT arm compare favorably to those of WP-CRT arm. Based on our results, we could suggest limiting V45 of the small bowel to <180 cc to reduce acute GI toxicities. However, we could not derive any definite statistically significant dosimetric parameter for chronic GI toxicities. There is emerging evidence from the EMBRACE group [25] that combined doses from external beam radiotherapy and brachytherapy could be a strong determinant of late rectal morbidities and they suggest limiting the D2cm3(combined dose received by 2 cc) of rectum to <65 Gy of EQD23 (2-Gray equivalent dose considering alpha/beta ratio for rectum to be 3).
Limitation
The limitation of our present study is small sample size. A longer follow-up would also be desirable for the reporting of chronic toxicities in our study. Magnetic resonance imaging-based image guided brachytherapy has become established in recent times and was not used in our study, since this was not routinely available. However, we would suggest incorporating this in future studies. PET-CT for baseline staging and for follow-up of cervical cancers (not done in present study) may also be integrated in future studies. We did not use bone-marrow constraints in our study, however, would suggest its inclusion in future studies particularly those using intensified chemotherapy regimens with WP-IMRT. The present analysis lacks statistical power to differentiate the survival outcome or chronic toxicity in between the two arms; however, the present study is the only study to date to have compared long term survival outcomes between WP-IMRT and WP-CRT arms in a randomised fashion and we feel that this could be a valuable addition to the present literature.
The interest and evidence in favor of use of WP-IMRT for the definitive management of LACC is growing day by day. Guidelines for target volume delineation have emerged [26-28] and the results of our study may further lend support to accrual in multicentric phase III randomized trial [23], the final results of which are eagerly awaited. Another area of active research is bone marrow sparing WP-IMRT in this setting, which would encourage the use of more intensified regimens [22] to improve the survival of LACC without escalating the haematological toxicities. Mell LK et al., in a phase II trial have shown a significant reduction in the incidence of Grade-3 or higher neutropenia with PET image guided IMRT [11]. Wider applicability of WP-IMRT in terms of machine and caregiver time would be a hurdle in resource constraint setting. Cost-effectiveness analysis should also be incorporated in future planning of similar studies.
Conclusion
The updates long term results at 5 years of our study which included 44 patients being treated with CCRT, continues to show non-inferior outcome of WP-IMRT as compared to WP-CRT with lesser chronic GI toxicities in WP-IMRT arm. Pending mature results from the phase III multi-institutional randomised trial, it seems reasonably safe for us to recommend WP-IMRT in the management of LACC.