Immunodeficiency syndromes whether congenital, spontaneously acquired, or iatrogenic are characterized by unusual susceptibility to infections. Immunosuppression can mask signs and symptoms of serious Gastrointestinal (GI) pathology; thus a high index of suspicion is needed to make an early diagnosis. This is accomplished by a variety of methods including direct demonstration of microorganisms and serological tests to detect antigen and specific antibodies. While stool examination may diagnose some pathogens, at times this may be negative and upper oesophagogastroduodenoscopy and colonoscopy findings maybe unremarkable [1-6]. Studies state that gastrointestinal biopsies from various sites were found to be useful in evaluating the aetiology of chronic diarrhoea. Some studies found upper GI biopsies to be more useful than the lower GI biopsies and some found that the combined biopsies from both upper and lower GI tract yielded better results [7,8]. The diagnostic features in these situations are often subtle, requiring systematic examination of multiple sections at high power and the use of special stains which are not otherwise performed [9]. This study was conducted to evaluate the usefulness of a duodenal biopsy in determining the aetiology of chronic diarrhoea in immunosuppressed patients not diagnosable by routine non-invasive methods.
Materials and Methods
This prospective study was done in the Department of Histopathology, Apollo Hospitals, Chennai, Tamil Nadu, India, over a period of 26 months (February 2010 to March 2012). Duodenal biopsies from 42 immunosuppressed patients (including Acquired Immuno Deficiency Syndrome (AIDS), transplant recipients and those with drug induced immunosuppression), who presented with chronic diarrhoea undiagnosable by routine non-invasive investigations were included in this study. The non-invasive investigations done include stool routine examination, stool culture and sensitivity, stool modified acid fast stain, stool Clostridium difficile assay and serum Cytomegalovirus (CMV) quantification. Exclusion criteria included cases where clinical details were not available, cases where duodenal biopsy was not part of the workup, and cases where history of immunosuppression was not ascertainable from clinical data. Clinical details were retrieved from the hospital medical records. At endoscopy the duodenum was biopsied, in addition to biopsies from other sites where indicated. Colonoscopy was performed in selective cases as deemed necessary and biopsies were taken if appropriate. Prior approval for the study was obtained from Institutional Ethics Committee- Clinical Studies (ECR/37/Inst/TN/2013/RR-16).
The samples were received in 10% neutral buffered formalin, processed in graded alcohols, cleared and embedded in paraffin wax, and 4 micron thick sections were cut and stained with Haematoxylin and Eosin stain. Special stains including Periodic Acid Schiff (PAS), Modified Masson’s Trichrome, Zeihl- Neelsen and Giemsa were performed when deemed necessary.
Results
Clinical Features and Investigations
This study includes duodenal biopsies from 42 immunosuppressed patients, of whom 31 were renal transplant recipients, five people with AIDS, four with autoimmune diseases and two liver transplant recipients. The duration of diarrhoea ranged from 4 weeks to 1 year. Diarrhoea was watery in 33 patients and semisolid in nine patients. Stool routine microscopy was unremarkable in 40 patients with the remaining two showing Giardia cysts and Entamoeba histolytica cysts. These two patients were subjected to endoscopy and biopsy due to lack of treatment response. Additional testing such as modified acid fast stain (17 cases), stool Clostridium difficle toxin assay (5 cases) and stool culture (10 cases) were done on selected cases and were negative. Peripheral blood Cytomegalovirus (CMV) DNA quantification was done on 12 patients of whom five patients had increased viral load. Upper GI endoscopy revealed mucosal abnormalities in only 27 patients (64.3%) and was unremarkable in rest of the cases. Colonoscopy was also done in 13 patients as part of the workup for the chronic diarrhoea. Mucosal abnormalities were noted in terminal ileum in three patients and in colon in two patients which were respectively biopsied. These findings are summarised in the following flow chart [Table/Fig-1].
Flowchart showing workup of the study.
Microscopic Features
An infectious aetiology was identified in 18 cases (42.9%). The most frequently found pathogens were protozoans (15 cases), followed by viruses (two cases) and helminths (1 case). The pathogens identified in the different immunosuppressive settings are listed in [Table/Fig-2] and are depicted in [Table /Fig-3,4].
Pathogens found in different immunosuppressive conditions.
| Type of Immunosuppression | Pathogens | Number of Cases |
|---|
| Renal transplantation | Microsporidia | 7 |
| Giardia | 3 |
| Cryptosporidia | 2 |
| Isospora | 1 |
| Cytomegalovirus | 1 |
| Liver transplantation | Cytomegalovirus | 1 |
| HIV/AIDS | Cryptosporidia | 2 |
| Autoimmune diseases | Strongyloides | 1 |
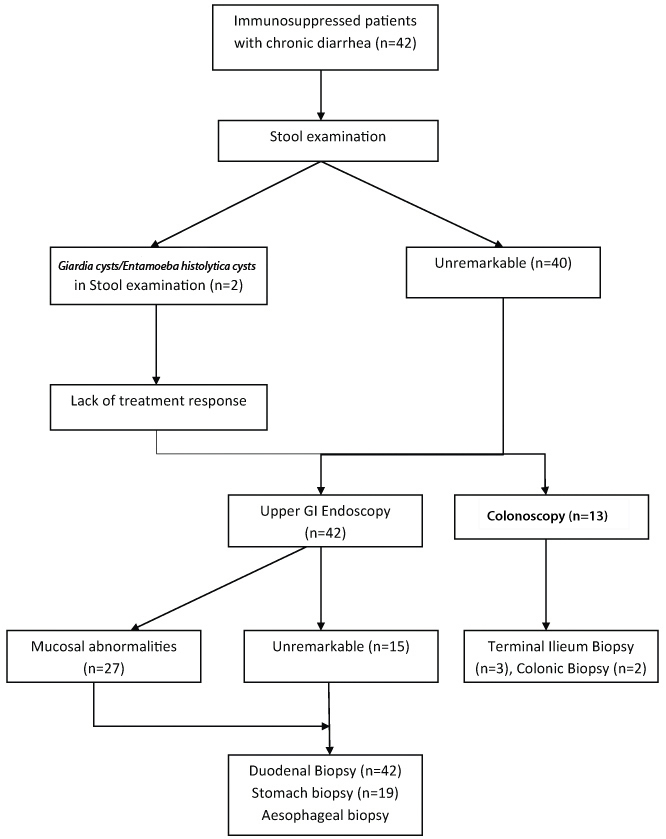
a&b) (H&E-X1000): Multiple plasmodia of Microsporidia within parasitophorous vacuole; c) (H&E-X400); d) (H&E-X1000): Cryptosporidia on the surface of epithelium; e) (H&E-X100); f) (H&E-X1000): Trophozoites of Giardia lamblia; g&h) (H&E-X 400): Microgametocytes, macrogametocytes and schizonts of Isospora belli.
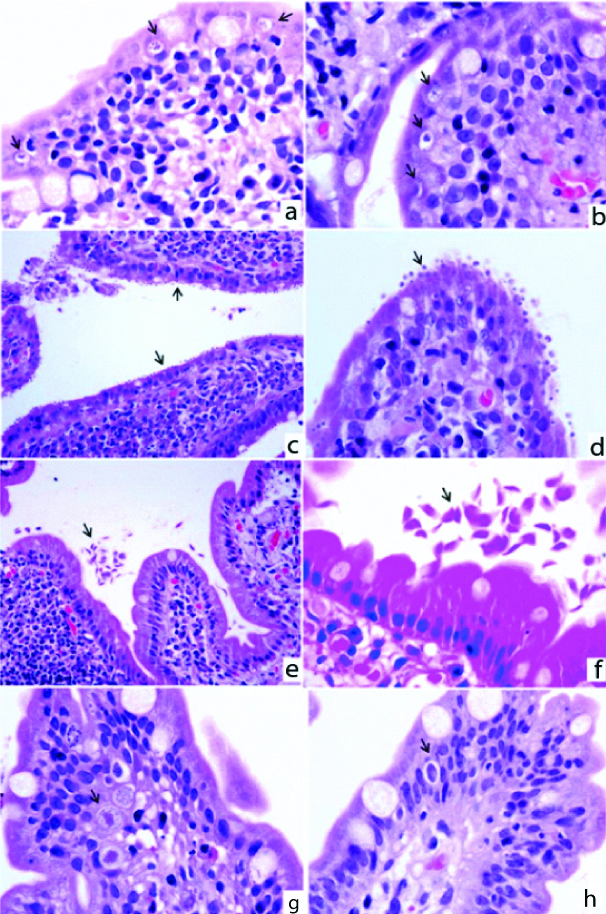
a&b) (H&E-X400): Cytomegalovirus inclusions within crypt epithelium and stromal cells; c&d) (H&E-X400): Adult worms and embryonated eggs of Strongyloides stercoralis.
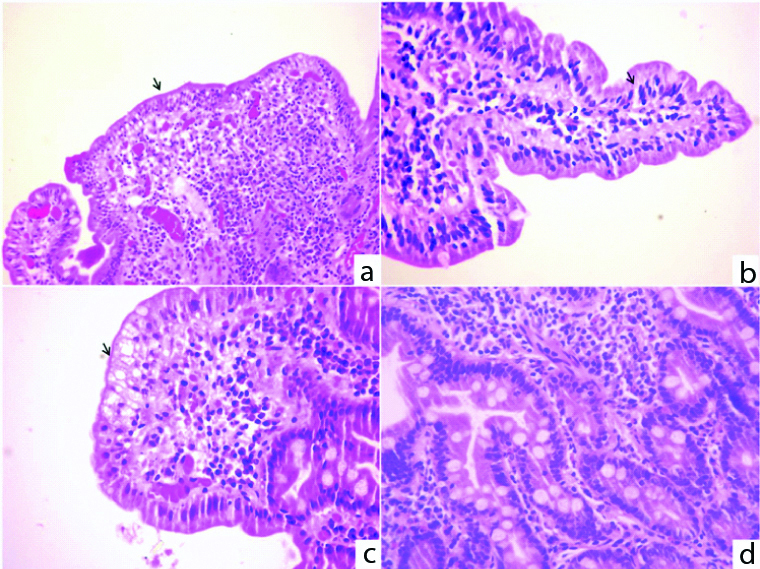
Architectural distortion was noted in the duodenal biopsy in 25 cases (59.5%), in the form of villous blunting, branching, broadening and fusion. Borderline intraepithelial lymphocytosis was noted in nine biopsies (25-29 intraepithelial lymphocytes/100 enterocytes) whereas definite intraepithelial lymphocytosis (>29 intraepithelial lymphocytes/100 enterocytes) was noted in eight biopsies. The surface epithelial abnormalities that were noted included intracytoplasmic vacuolation (15 cases), apoptotic changes (two cases) and ulceration (two cases). Increased lamina propria cellularity by inflammatory cells was present in all cases, ranging from mild to severe. These changes are depicted in [Table/Fig-5].
a) (H&E-100X): Villous blunting; b) (H&E-400X): Increased intraepithelial lymphocytes; c) (H&E-400X): Cytoplasmic vacuolation;d) (H&E-400X): Increased lamina propria cellularity.
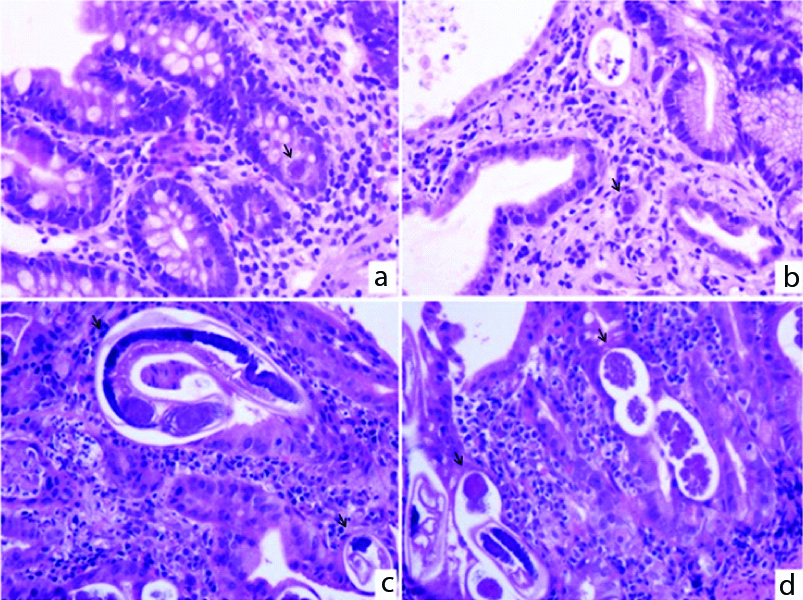
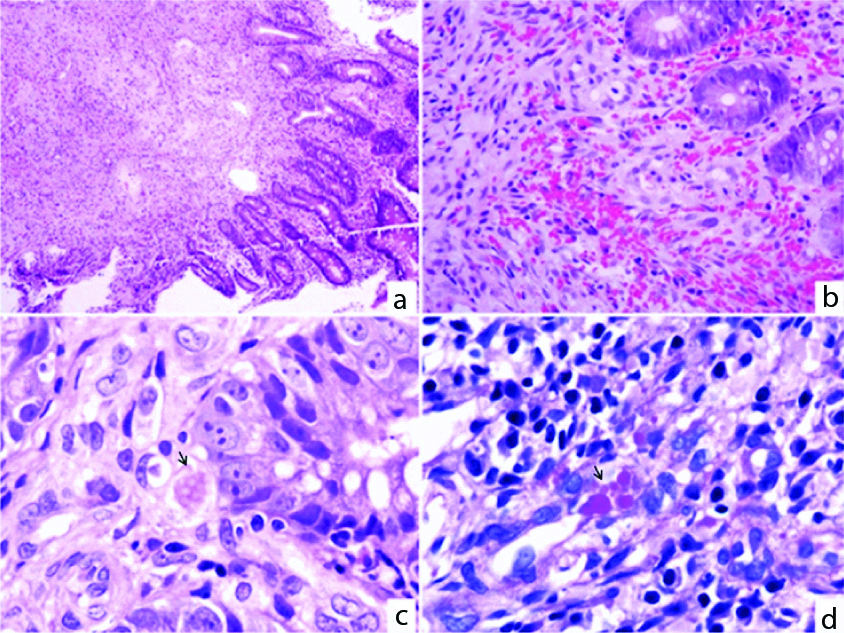
In one patient with HIV/AIDS the duodenal biopsy revealed an atypical spindle cell proliferation with extravasated erythrocytes, haemosiderin deposits, haemosiderophages and PAS positive hyaline globules morphologically consistent with Kaposi sarcoma [Table/Fig-6]. Gastric and colonic biopsies of the same patient also had Kaposi sarcoma.
a) (H&E-X 100); b) (H&E-X 400): Duodenal mucosa with lamina propria showing atypical spindle cell proliferation with numerous extravasated erythrocytes; c) (H&E-X 1000); d) (PAS-X 1000): Hyaline globules are noted which is highlighted by PAS.
Other GI Biopsies
In addition to duodenal biopsies, biopsies from other sites in the GI tract were performed, wherever indicated. These included oesophageal biopsies in 10, gastric biopsies in 19, ileal biopsies in two and colonic biopsies in three patients. A variety of pathogens were discovered; these are summarised in [Table/Fig-7].
Pathogens identified in other GI biopsies.
| Biopsy site | Total number of cases | Diagnosis |
|---|
| Oesophagus | 10 | 4 Candida, 3 CMV |
| Stomach | 19 | 2 CMV, 2 Cryptosporidium & CMV, 1 CMV & H pylori,2 H pylori, 1 Kaposi sarcoma |
| Terminal ileum | 2 | 1 Histoplasma1 Cryptosporidium |
| Colon | 3 | 1 CMV, 1 Histoplasma,1 Kaposi sarcoma |
The duodenal biopsy, when considered by itself yielded an aetiological diagnosis for the chronic diarrhoea in 19 cases (45.24%) while combining it with other GI biopsies (oesophageal, gastric, ileal and colonic) increased the diagnostic yield (64.29%). Multiple infections were noted in one patient in the duodenal biopsy alone whereas when additional GI biopsies were included, multiple infections/ infestations were observed (six cases).
Discussion
Chronic diarrhoea is an important clinical problem in immunocompromised patients. Post-transplant diarrhoea may lead to inconsistent immunosuppressive drug levels; thereby enhancing the risk of graft loss. When stool examination does not identify a pathogen, the decision to undertake a more aggressive diagnostic evaluation is still controversial. Many studies have been done to evaluate chronic diarrhea in the different settings of immunosuppression including HIV/AIDS [7,8,10-14], solid organ transplant recipients [15-17] and therapeutic immunosuppression [18,19]. Though these have focused on the epidemiology of enteric pathogens in chronic diarrhoea; diagnostic yield of stool examination and endoscopic evaluation; and diagnostic yield and cost effectiveness of GI endoscopies, etc.
Earlier studies have compared the usefulness of different biopsy sites to determine the cause of chronic diarrhoea in different immunosuppressed populations. We chose duodenal biopsies as preparation is easier and it is less expensive. A study by Weber R et al., showed that upper endoscopy with biopsy had a slightly higher diagnostic yield than ileocolonoscopy with biopsy in the evaluation of chronic diarrhoea in HIV patients [7]. However, a study by Bini EJ et al., found that the diagnostic yield of upper GI endoscopy with biopsy was 29.6% in HIV patients which was lower when compared with colonoscopy and biopsy (38.7%). They found the diagnostic yield was around 47.9% when multiple biopsies were taken from both upper and lower gastrointestinal tract [8]. Overall there is a consensus that combining biopsies from multiple sites is the most rewarding method of diagnosing the aetiology of chronic diarrhoea in a setting of immunosuppression [11]. In our study, we were not able to compare the effectiveness of a duodenal biopsy versus other GI biopsies as the latter were taken inconstantly.
Blanshard C et al., had investigated chronic diarrhoea in 155 AIDS patients and found that the stool examination had a higher sensitivity (47%) when compared to other investigations like duodenal biopsy (44%), jejunal biopsy (38%) and rectal biopsy (33%). They also found that the diagnostic yield increased when up to six stool specimens (47%) were examined when compared to three (39%) or single stool sample (19%) examinations. They concluded that no investigation is 100% sensitive for identification of any pathogen [12]. Similarly Weber R et al., showed that the stool examination is as sensitive as upper gastrointestinal endoscopic evaluation for all pathogens except CMV and Leishmania [7]. In the present study the yield from stool examination was necessarily negative as this was an inclusion criterion and we did not assess comparative sensitivity of stool examination versus biopsy. Modified acid fast staining had been done on stool samples from 17 patients in this study to look for Cryptosporidium, Isospora and Cyclospora oocysts and was found to be negative. Though the stool examination by Modified Acid fast staining was negative, we found Cryptosporidiosis and Isosporiasis in two duodenal biopsies. This lack of sensitivity is explained by the inconsistent staining by modified acid fast stain, especially when the organism burden is low or the organisms may be intermittently shed [20,21].
Studies state that biopsies even from a normal appearing mucosa yield positive results especially in immunosuppressed individuals similar to our study [2-4]. However, in cases with positive endoscopic findings, the biopsy was almost always abnormal. Thus, the endoscopic findings create a high index of suspicion, thereby playing an important role in identifying pathogens.
The duodenum is a good site for biopsy in the investigation of chronic diarrhoea in a setting of immunosuppression. In this study a diagnostic yield of a duodenal biopsy alone was 45.24%. Combining duodenal biopsy with biopsies from other sites in the GI tract enhances the diagnostic yield moderately (64.29% as against 45.24%). Multiple infections were seen in 14.29% of patients reflecting the need for a diligent search even after one pathogen is found. It should be kept in mind that even malignancies such as Kaposi sarcoma which can be diagnosed only by histopathologic examination, can present as chronic diarrhoea.
Limitation
In the cases studied, we were unable to establish a diagnosis of other conditions presenting with chronic diarrhoea (such as malabsorption states) with confidence as there are overlapping features with drug induced changes and the nonspecific changes accompanying infectious processes. Hence, the duodenal biopsy is a good site for specific pathogens, but its role in detecting the changes due to other factors such as drugs, direct HIV effect, etc., is less clear. The small cohort size of this study precludes more for reaching conclusions. Also, the sensitivity of the duodenum as a biopsy site in reflecting the cause of chronic diarrhoea could not be assessed as matched biopsies from other GI sites were not performed in all cases.
Conclusion
While this study involved a relatively small number of patients, the results clearly indicate that the duodenal biopsy plays a significant role in establishing a specific diagnosis in around half the cases. Multiple endoscopic biopsies from various sites have increased the diagnostic yield. Organisms such as Microsporidia and Cryptosporidia may be missed unless very carefully looked for, and interaction with the clinician, familiarity with morphology and a high index of suspicion help in arriving at a correct diagnosis.