Informed consent is essential in daily clinical practice, particularly for legal, ethical and administrative purposes [1]. It is important to obtain informed consent before a surgical procedure, which may be achieved in a two-way and balanced discussion between the clinician and patient [2]. This process in essence protects the patient’s autonomy in the decision-making [3]. Therefore, the clinician must ensure that the patient is adequately informed regarding the proposed treatment before embarking on it [4].
MTMS is a common surgical procedure performed routinely by dental practitioners. The most serious complications associated with this surgery are injuries to the inferior alveolar nerve and lingual nerve. Clinicians are trained to explain these complications and others thoroughly before surgery. Informed consent is traditionally obtained verbally. However, the use of other media, i.e., audiovisual material, a leaflet or decision aids have been described either alone or as a supplement to the common verbal explanation [5].
Written leaflets is the most common method used to supplement the verbal information given in improving recall of information given during the informed consent process [5]. Previous studies have shown improved information recall with the use of written information sheet to supplement verbal information [6,7]. There were, however, a number of studies that showed no difference compared to the verbal methods [8,9]. Specific to MTMS, previous study suggested that the addition of written material improved patient’s knowledge [10].
The study aimed to investigate the difference in the recall of complications of the MTMS between the verbal only versus combined verbal with written consent interventions. We also compared the satisfaction among patients with regard to the process of obtaining informed consent.
Materials and Methods
The design of this research was a single-blind, randomised controlled study. This study was carried out in the Oral and Maxillofacial Surgery Clinic, Faculty of Dentistry Universiti Kebangsaan Malaysia from April 2014 to February 2015. The faculty is located in the city centre of Kuala Lumpur, the capital city of Malaysia and receives any patient that is referred to or walk-ins. This study received institutional ethical approval {UKM 1.5.3.5/244/DD/2014/010(1)}. The methodology adhered strictly to the Declaration of Helsinki guidelines.
The trial was not registered in any of the clinical trial database. It was not necessary because the trial did not study “the relationship between a health-related intervention and a health outcome”.
Subjects
Subjects who met the following criteria: age ≥18 years, diagnosed with mandibular third molar that required surgical intervention, fit to undergo the surgical procedure (healthy or well-controlled systemic disease) and able to understand English and/or Malay language were included in the study. In contrast, the exclusion criteria were subjects with a cognitive disability, inability to understand both English and Malay language and refusal to take part in the study at any point.
The sample size was calculated using a method obtained from the Open Epi software version 3.01, and the calculation was based on the previous study by Chan Y et al., [14]. Power analysis revealed that in order for an effect of this size to be detected (80% power) as significant at the 5% level, a sample size of 138 subjects would be required.
Randomisation
Simple randomisation of subjects was performed based on the random number table. Two groups of intervention were formed and researchers were blinded from the type of intervention until the subjects completed the three phases of the interview. A research manager (not blinded) was appointed to allocate subjects into the Verbal (V) or combined Written-Verbal (WV) groups.
Grouping and assessment
The groups were:
VW group: subjects who were given a written and verbal explanation of the procedure and complications.
V group: subjects who were given verbal explanation only of the procedure and complications.
The V group was used as the control group as the verbal explanation in the informed consent process is the standard practice (usual care) of taking consent. As observed in a systematic review by Kinnersley P et al., 62.5% (45 of 72) of the included trials in their study used verbal explanation as the control group [5]. The verbal method is the standard practice in the informed consent process, therefore, it would be regarded as the ideal control.
All the written and verbal materials were prepared in both English and Malay language. The translation for verbal information text, written information leaflets, consent form and research tools (question on demographic, satisfaction and recall of information) were performed by one of the authors and checked by a certified translator from the Malaysian National Institute of Translation. The translator also performed back translation. The verbal explanation and signing the consent form was given according to the subject’s preferred language by a single researcher throughout the study. The information leaflets (for VW groups) had both languages in a single page. The researcher (MMY) used a standardise text as a guide for the verbal information to the subjects to minimise bias in providing the information.
The groups were assessed based on their recall of seven specific complications in relation to MTMS. The seven possible specific complications are listed below [15]:
Paraesthesia of the lower lip
Lingual paraesthesia
Facial oedema
Trismus
Pain
Allergic reactions
Infection
The score of the recall was categorised into poor score (0-2 complications recalled), moderate score (3-5 complications recalled) and good score (6-7 complications recalled).
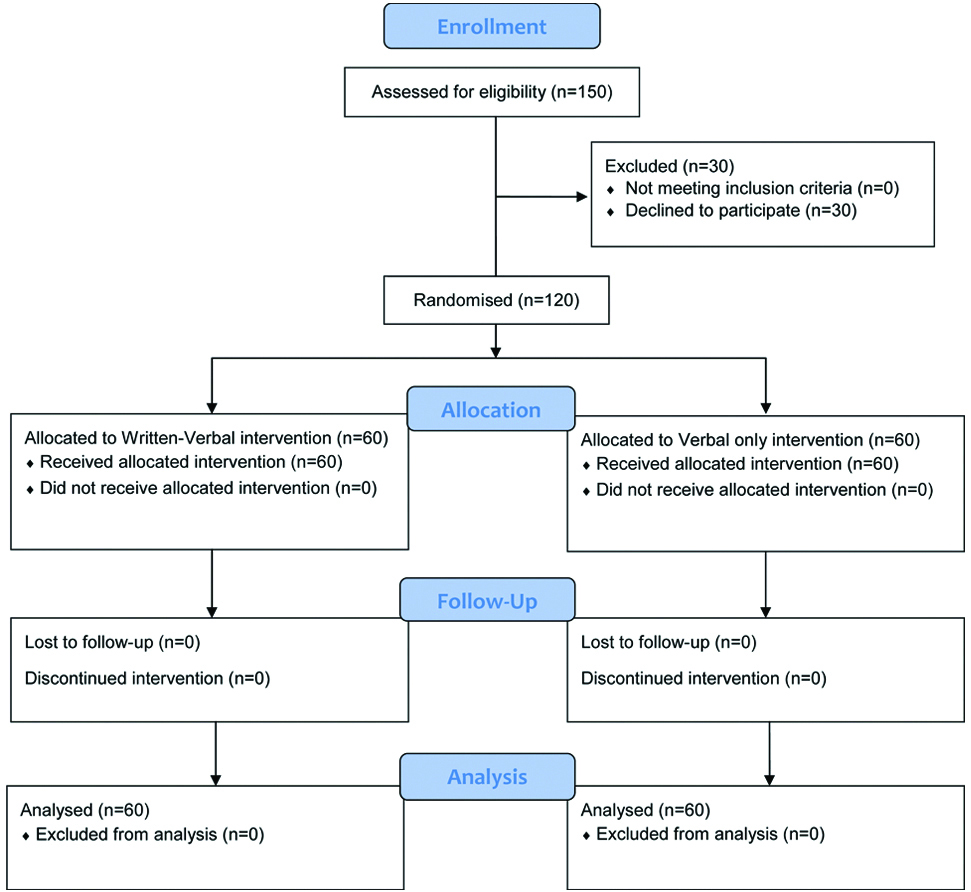
Three phases were used to assess the recall of the seven complications, i.e., the first consultation phase (first phase), the preoperative phase (second phase) and the postsurgery phase which was scheduled a week following the surgery (third phase). During the first consultation, all subjects were assessed by one of the researchers. The seven specific complications were explained to both groups verbally by the same researcher to ensure uniformity of delivery. Subjects of the VW group received additional information leaflets about the seven complications without the knowledge of the researchers. The first recall response was recorded after the consultation session. In the preoperative assessment, the second recall of the seven complications was performed just before the informed consent of the surgery. Assessment of the last recall was conducted a week after surgery. Satisfaction on the information received was assessed during the postsurgery phase. All assessments were made by one researcher. The flow diagram of the research conduct is illustrated in [Table/Fig-1].
Consort Flow Diagram for the study conduct.
Research Tools
Two main research tools were used in this research. Each was prepared in both English and Malay language. The tools were:
The research proforma comprised information about the demographic and satisfaction responses on the informed consent form. Satisfaction responses were categorised into satisfied with the process, satisfied but still need to look for additional information and not satisfied.
The recall response datasheet was prepared based on the seven complications described by Ferrús-Torres E et al., [15].
Ten clinicians consisting of specialist and trainee specialists performed all MTMS. Cases with close proximity of the third molar to the mandibular canal were performed by specialists according to the standard clinical protocol. Preoperative assessment, surgery and postoperative review were performed by the designated specialist/trainee on the case.
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) version 12.0 (IBM SPSS, Chicago, IL, USA) was used for data entry and analysis. Descriptive statistics in the form of frequency and percentage, mean with Standard Deviation (SD) and median with Inter-Quartile Range (IQR), were calculated where appropriate. The association between the score of recall and interventions was assessed using the Fisher’s-exact test. The Friedman test was conducted to compare the percentage of recall between the first, second and third phases. In addition, the Wilcoxon signed-rank test was performed to compare the percentage of recall response (median) between the first and second phases, second and third phases and first and third phases. The level of significance was set at 0.05.
Results
Initially, 150 subjects were screened. However, only 120 subjects fulfilled the inclusion criteria and were recruited into this study. These subjects provided their written consent prior to taking part in this study. Next, subjects were distributed equally and randomly into two groups, the V and VW groups. There were 47 male (39.2%) and 73 female (60.8%) subjects with the age of the subjects ranging between 18 to 42 years. Details of the subjects’ demographics are shown in [Table/Fig-2].
Demographics and level of satisfaction of the study population.
| Demographics | Intervention | Total |
|---|
| Verbal and written | Verbal only |
|---|
| n (%) | n (%) | n (%) |
|---|
| Gender |
| Male | 24 (20.0) | 23 (19.2) | 47 (39.2) |
| Female | 36 (30.0) | 37 (30.8) | 73 (60.8) |
| Age (in years) |
| Mean±standard deviation | 27.3±5.3 | 27.0±5.3 | 27.2±5.3 |
| Age categories |
| 18−25 years | 23 (19.2) | 24 (20.0) | 47 (39.2) |
| >25 years | 37 (30.8) | 36 (30.0) | 73 (60.8) |
| Ethnicity |
| Malay | 48 (40.0) | 44 (36.7) | 92 (76.7) |
| Chinese | 10 (8.3) | 15 (12.5) | 25 (20.8) |
| Indian | 1 (0.8) | 1 (0.8) | 2 (1.7) |
| Others | 1 (0.8) | 0 (0.0) | 1 (0.8) |
| Education |
| Secondary | 18 (15.0) | 23 (19.2) | 41 (34.2) |
| Tertiary | 42 (35.0) | 37 (30.8) | 79 (65.8) |
| Satisfied with the current information |
| Yes | 58 (48.3) | 59 (49.2) | 117 (97.5) |
| No | 2 (1.7) | 1 (0.8) | 3 (2.5) |
| Sought further information |
| Yes | 9 (7.5) | 8 (6.7) | 17 (14.2) |
| No | 51 (42.5) | 52 (43.3) | 103 (85.8) |
Knowledge about informed consent: Almost all subjects, (n=118, 98.3%) were aware and understood about the practice of informed consent prior to any surgical procedure in a healthcare service. One subject from each intervention group (n=1, 0.8% for each group; total n=2, 1.7%) claimed to have no knowledge of informed consent.
Satisfaction with information provided: Lastly, subjects were assessed on their satisfaction with the content of the consent [Table/Fig-2]. Overwhelming majority of the subjects (97.5%) was satisfied with the information they received regarding the MTMS with similar satisfaction among both groups. On seeking further information on the proposed procedure, only 14.2% of the subjects did so with almost equal representation from both groups.
Recall of complications: The results of recall of complications are shown in [Table/Fig-3]. It showed a decrease in recall according to phase in both interventions. Fisher’s-exact test showed no statistically significant difference between the two intervention groups according to phase [Table/Fig-3].
Scores of the recall response of complications.
| Recall of complication scores and percentage | Group | Fisher’s Exact Test p-value |
|---|
| VW Intervention (n=60) n (%) | V Intervention (n=60) n (%) |
|---|
| First Phase | Scores | Poor (0-2) | - | 1 (0.8) | 0.43 |
| Moderate (3-5) | 44 (36.7) | 39 (32.5) |
| Good (6-7) | 16 (13.3) | 20 (16.7) |
| *Percentage (%) | Mean±SD | 69.77±15.80 | 68.57±19.99 |
| Median (IQR) | 71.43 (28.57) | 64.29 (28.57) |
| Second Phase | Scores | Poor (0-2) | 1 (0.8) | 1 (0.8) | 1.00 |
| Moderate(3-5) | 48 (40.0) | 49 (40.8) |
| Good (6-7) | 11 (9.2) | 10 (8.3) |
| *Percentage (%) | Mean±SD | 64.05±15.45 | 65.00±14.98 |
| Median (IQR) | 57.14 (14.29) | 71.42 (32.14) |
| Third Phase | Scores | Poor (0-2) | 1 (0.8) | - | 0.79 |
| Moderate (3-5) | 51 (42.5) | 53 (44.2) |
| Good (6-7) | 8 (6.7) | 7 (5.8) |
| *Percentage (%) | Mean±SD | 64.05±14.04 | 62.38±13.65 |
| Median (IQR) | 64.29 (14.29) | 57.14 (14.29) |
For the individual group assessment, the Friedman test and the Wilcoxon signed-rank test were performed [Table/Fig-4,5]. Comparison of the repeated measures using the Friedman test showed a statistically significant difference for the VW group, p=0.02 but not for the V group, p=0.53 [Table/Fig-4].
Comparison of the percentage of recall for the two intervention groups.
| Intervention | Percentage of recall of complications median (IQR) | Friedman test p-value |
|---|
| First phase | Second phase | Third phase |
|---|
| VW | 71.43 (28.57) | 57.14 (14.29) | 64.29 (14.29) | 0.02 |
| V | 64.29 (28.57) | 71.42 (32.14) | 57.14 (14.29) | 0.53 |
VW: Verbal and written; V: Verbal
The Wilcoxon signed-rank test showed a statistically significant difference between the first and second phases (Z= −2.50, p=0.01) and between the first and third phases (Z= −2.55, p=0.01) in the VW intervention. In the V intervention, there was a statistically significant difference between the first and third phases (Z= −2.11, p=0.04) [Table/Fig-5].
Comparison between the phases for the two intervention groups.
| Intervention | Wilcoxon signed ranks test, Z and p-value |
|---|
| First phase vs Second phase | Second phase vs Third phase | Third phase vs First phase |
|---|
| Z | p-value | Z | p-value | Z | p-value |
|---|
| VW | -2.50 | 0.01 | -0.12 | 0.90 | -2.55 | 0.01 |
| V | -1.15 | 0.25 | -1.17 | 0.24 | -2.11 | 0.04 |
VW: Verbal and written; V: Verbal
Discussion
Informed consent is considered valid when it incorporates five elements, which are volunteerism, capacity, disclosure, understanding and decision [16]. An individual who gives consent to a procedure must be able to appreciate that the information provided is of personal relevance and he/she should retain and understand the content, weigh the consequences of different decisions, ask for clarification and communicate decisions [17]. In this study, it was opted to use the MTMS informed consent process because it is considered an ideal clinical model in dentistry [15]. This is due to MTMS being the most frequently performed surgery with common surgery-related complications (pain, swelling, infection etc.,) and medico-legal associated complications (inferior alveolar and lingual nerve injury) [15]. Furthermore, by limiting cases to only mandibular third molar needing surgical intervention, uniformity is achieved thus ensuring the type of procedure would not be a confounding factor in the results. This study was further limited to adult patients because patient aged below 18-year-old would need parental consent thus the assessment of recall, perception and satisfaction would not represent the patients’ view directly. These measures were taken to ensure validity of the study results.
Randomised controlled trial was performed to provide the best empirical evidence on this topic. The hallmark of randomisation is that it prevents allocation bias thus produce comparable groups with equal known and unknown confounding factors. An example of a potential confounding factor is the type or depth of third molar impaction. Certain impaction has increased risk for inferior alveolar nerve injuries and this could increase awareness of patient. Randomisation would distribute this clinical factor equally in both groups thus rendering it as a non-contributing factor. Furthermore, standardisation of content and the operator in the intervention was performed to prevent increased awareness of subject in this regard. Other clinical factor that could possibly be a confounding factor is the skill of operator in which, less skilled operator could produce more complication thus would possibly create awareness among subjects that develop that complication specifically. This may affect the postsurgery phase recall assessment. Variability factor was reduced by restricting the operators to only qualified oral surgeon and trainee oral surgeon (postgraduate student) in which MTMS is the routine procedures for this group of operators. Furthermore, randomisation would then distribute equally among groups any remaining differences of skills among operator.
To compare the success or effectiveness of the informed consent process, several parameters have been used as a metric to measure its outcome [5]. Among the parameters used to determine informed consent effectiveness include by measuring patient’s understanding, recall of knowledge, deliberation and communication of decision [5]. The ability to recall information is the most commonly used, arguably due to it being the most convenient and provides easily measurable (objective) outcome. This Our study found similar recall scores involving all three phases between the two groups suggesting similar effectiveness between V compared to VW intervention. Previous studies has found conflicting outcome when these two interventions were compared [6-8,10,18]. Synthesis of results from multiple randomised controlled trials however shows the supplementation of written information does improve the recall rate [5]. The differences between studies on the effectiveness of the addition of leaflets are likely due to the variation such as different patient population, procedure/surgery types (complex versus simple), the amount and design of information in the leaflets.
The present study also investigated the effect of time to the recall rate. We observed a decline in good recall scores in both groups especially from the first (immediate) to the third (postoperative) phases. This implies that in both interventions, the retention of information suffers attrition over time. This expected finding was similar to other studies [19,20]. Herz DA et al., reported an immediate recall of 43.5% that declined to 38.4% six weeks after the consent interview [19]. In addition, Godwin Y reported a drop in the recall to 25.0% six days following a mammoplasty procedure [20]. This situation poses a conundrum, as ideally information of the procedure should be given days to weeks before the procedure as to allow the patient ample time to think and consider the proposed treatment. However, in practice, getting consent on admission (inpatient) or immediately before (outpatient) procedure is usually performed. This short period may have the benefit of good knowledge but then limits the time to decide.
It was observed that 97.5% of subjects expressed their satisfaction with the information received. Interestingly, satisfaction level was equal in both V and VW intervention. Furthermore, the number of patient seeking further information was also similar in both groups. This result was not surprising as patients’ satisfaction was believed to be related to interactions during both the discussion and decision-making process rather than from the provided information [21]. This notion was supported by a finding in another study where the majority of their patients were satisfied with the process of informed consent despite having a poor understanding [13]. A high level of patients’ trust in their surgeons also might further lead to less desire for additional information but this may result in inaccurate expectations and dissatisfaction with the outcomes [22]. Other explanation included the fact that patients who have already decided to have a surgery, such as the case of problematic wisdom tooth, were less likely to listen to, research or find more information about the potential complications [22].
Limitation
There were four main limitations observed while conducting this study. First, 30 subjects refused to participate in this study thus meeting the exclusion criteria. The reason given was the time factor, as they had to participate in three consecutive interviews. Second the design of the study. The postsurgery (third phase) assessment of recall may be of little value since the surgery has already been completed. The focus of the postsurgery evaluation, apart from the satisfaction assessment, should be on the comparison of information gained preoperatively with the actual experience during the surgery. Thirdly, although randomisation should have prevented systematic differences in allocation of subjects, these clinical parameters (type of impaction and the skill of operators) were not recorded and analysed, thus we could not show that the distribution was totally equal. Lastly, the sample size can be improved. Further trials with a larger sample size should be conducted to reflect the actual clinical situation.
Conclusion
Both interventions, the verbal and verbal supported by an information leaflet, were equally acceptable in providing sufficient information during informed consent. Improvement towards understanding the entire process and providing more targeted materials about each surgery, however, need to be both addressed appropriately.
Ethical approval: Ethical approval was obtained from the institution ethical committee. The Universiti Kebangsaan Malaysia ethics reference number are UKM 1.5.3.5/244/DD/2014/010(1).
Patient consent: Written consent was obtained from all subjects participating in this study.
VW: Verbal and written; V: Verbal
VW: Verbal and written; V: Verbal