Is Interleukin-18 an Early Diagnostic Biomarker in Contrast Induced Nephropathy?
Mohammed Mujahid1, Mohammed Shobha2
1 Associate Professor, Department of Physiology, Maheshwara Medical College, Isnapur, Hyderabad, Telengana, India.
2 Associate Professor, Department of Biochemistry, Maheshwara Medical College, Isnapur, Hyderabad, Telengana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mohammed Shobha, Chitkul, Isnapur, Hyderabad, Telengana, India.
E-mail: cardia1500@gmail.com
Introduction
The rise in number of cases of hospital-acquired acute kidney injury in patients undergoing diagnostic and therapeutic procedures by the use of contrast medium is a growing concern among nephrologists. Despite being a delayed marker, the measurement of creatinine level is one of the most popular methods in medical fraternity, to identify the degree of damage caused to the kidneys. Therefore there is a need for development of method for early detection and treatment of the contraindication of contrast media. Since serum Interleukin-18 (IL-18) levels rise early in the disease course, it may be a promising novel biomarker and an early indicator of tubular damage as compared to creatinine and other novel biomarkers.
Aim
The current study investigated the early rise in serum IL-18 after contrast induction.
Materials and Methods
Randomly selected 30 male wistar rats were given 0.6 mL of contrast iohexol (325 mg of iodine per mL) intraperitoneally and blood samples were collected before and after the induction of contrast by bleeding retro-orbital plexuses under isoflurane (USP) inhalation anaesthesia. Blood samples were collected at 3 hours, 6 hours, 12 hours, 24 hours and 48 hours of post-contrast administration. Results were analysed by using paired Student’s t-test and p-value<0.05 was considered to be statistically significant before and after contrast induction.
Results
Statistically significant increase in IL-8 levels at 3 (p<0.01), 6 (p<0.001), 12 (p<0.01) and 24 (p<0.01) hours pre- and post-contrast treatment was observed. However, no statistical difference was found at 48 hours pre- and post-contrast treatment (p>0.07).
Conclusion
The present study reveals an increase in IL-18 levels from 48% to 100% at 6 hours post-contrast induct in comparison to other standard markers like creatinine levels which increases at 48-72 hours post-contrast insult, revealed by the literature. Therefore it can be concluded that IL-18 is a better early novel biomarker for tubular damage assessment, after contrast insult.
Acute kidney injury, Cystatin C, Kidney injury molecule-1, Neutrophil gelatinase-associated lipocaline
Introduction
Rise in use of iodinated contrast by the medical fraternity in diagnosis and treatment has taken upper hand in recent decade but its effects on kidney functioning are questionable. So, there is a need to identify the early damage caused to the kidneys due to the use of iodinated contrast media. Although conventional biomarkers like urea and creatinine are used to detect the damage to the kidneys, but the rise in creatinine and urea levels occurs when there is a considerable loss of kidney functions [1]. Injection of iodinated contrast may cause ischaemia to the kidneys as it brings about vasoconstriction of renal blood vessels [2]. It is necessary to evaluate the early detection of kidney injury by novel biomarkers such as Neutrophil Gelatinase-Associated Lipocalin (NGAL), Cystatin C, Kidney Injury Molecule -1 (KIM-1) and IL-18 which have been proven to be specific for kidney injury. The current study investigates the early rise of serum IL-18 on contrast insult to the kidneys, so that early preventive measures can be adopted to avoid further loss of kidney function.
Materials and Methods
The study was conducted in December 2016 at the Department of Research and Development, Saveetha University, Chennai, India, and ethical clearance was obtained from Department of Research and Development, Saveetha Medical College, Saveetha University (SU/BRULAC/RD/ 001/2014). Thirty male Wistar rats weighing about 160 g were selected randomly, obtained from Biomedical Research Unit and Laboratory Animal Centre (BRULAC), Saveetha University, Chennai and divided into five different groups (n=6) sample size was calculated by resource equation (E) where confidence interval (CI) was 95% with 80% power of the study. There was no attrition or death of animal in this study, therefore the attrition sample size calculation was neglected.
Animals were maintained at standard laboratory conditions in wood shavings lined cages with food and water ad libitum throughout the experimental protocol. Blood samples were collected by bleeding retro-orbital plexuses under isoflurane (USP) inhalation anaesthesia before and after the induction of contrast. Animals were allowed to stabilise for 24 hours before the administration of contrast. About 0.6 mL of contrast (Iohexol) was injected intraperitoneally for each animal dose was calculated on the weight of the animal in accordance with contrast dose given to humans during diagnostic and therauptic procedures per kg body weight.
Pre- and post-contrast blood samples were collected at 3 hours, 6 hours, 12 hours, 24 hours and 48 hours post contrast, centrifuged at 3000 rpm (37°C) for 15 minutes (Rotofix 32A, Hettich) to obtain clear supernatant serum and was stored at -20°C until further analysis. IL-18 before and after contrast was assessed by using sandwich Enzyme-Linked Immunosorbent Assay (ELISA) (RayBio, Rat IL-18 ELISA, Catalog #: ELR-IL18). The optical density (OD) was measured spectrophotometrically at a wavelength of 450±2 nm. The concentration of IL-18 in the sample was proportional to the OD values and was calculated by comparing the OD of the samples to the standard curve.
Statistical Analysis
GraphPad Prism version 7.0 was used to calculate p-value and p<0.05 was considered to be statistically significant. Paired t-test was used to compare the data, before and after administration of contrast in rats. Data were presented as mean±SE.
Results
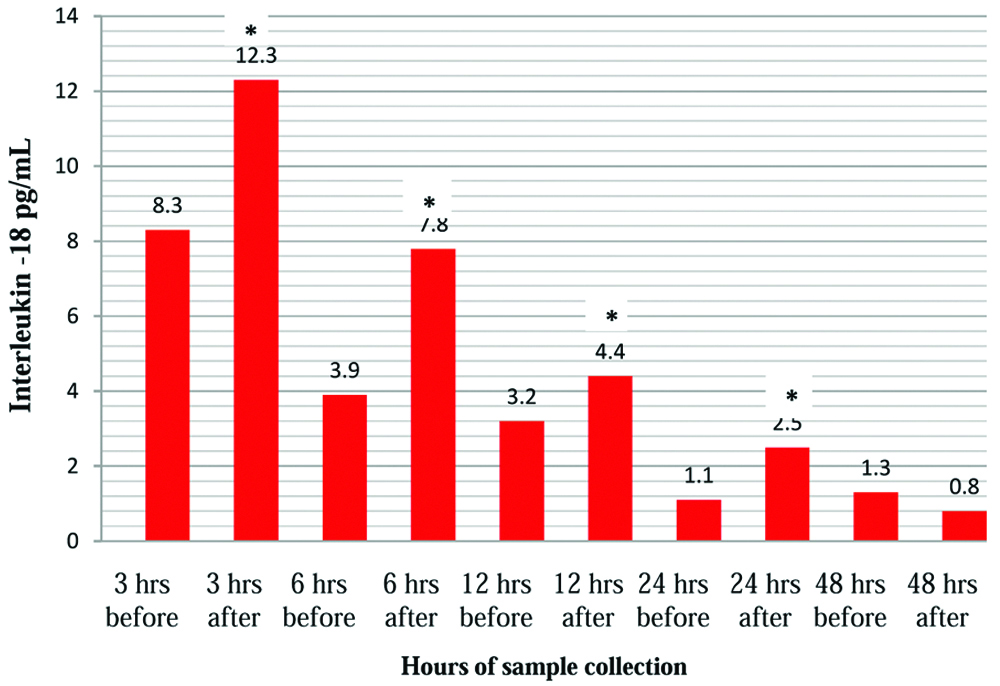
In the current study, the serum IL-18 levels increased by 48% at 3 hours, 100% at 6 hours, 37% at 12 hours, and 18% at 24 hours. The percent increase in the IL-18 levels suggests an early ischaemia of the renal tubular tissue upto 24 hours of contrast induction. Decrease in IL-18 at 48 hours may indicate the auto regulatory mechanism which has countered the ischaemia [Table/Fig-1,2].
Concentration of IL-18 before and after induction of contrast in rats
| Time Duration | IL 18 concentration (pg/mL) | p-value |
|---|
| Before contrast | After contrast |
|---|
| 3 hours | 8.3±0.8 | 12.3±4.5* | <0.01 |
| 6 hours | 3.9±1.6 | 7.8±2.1* | <0.001 |
| 12 hours | 3.2±1.4 | 4.4±1.1* | <0.01 |
| 24 hours | 1.1±0.3 | 2.5±1.0* | <0.01 |
| 48 hours | 1.3±0.2 | 0.8±0.4 | >0.07 |
Mean±SE (n= 6 in each group). *p-values<0.05, statistically significant for paired Student’s t-test.
Changes in serum interleukin-18 of male Wistar rats following contrast medium.
*p-values<0.05* statistically significant for paired t-test.
Discussion
Contrast Induced Nephropathy (CIN) is a growing concern among nephrologists, is one of the hospital-acquired diseases on rise, 10%-15% of cases in total Acute Kidney Injury (AKI) detected a year [3]. Use of contrast media brings about vasoconstriction of renal blood vessels and causes medullary hypoxia. In a prospective cohort study, CIN occurred in 11% of patients who underwent Computed Tomography (CT) by the administration of contrast media [4]. Use of conventional biomarkers like urea and creatinine shows increased levels in serum when there is a considerable loss of kidney function or damage [5]. The novel biomarkers like NGAL, KIM 1, Cystatin C are establishing their presence in diagnosing (AKI), but all of them have failed to detect AKI early. Novel biomarkers have several potential advantages which can detect the AKI [3]. Measurement of these novel biomarkers in serum may direct the nephrologists attention to ongoing kidney damage before measurable cause of kidney dysfunction occurs. Its early detection plays an important role in discontinuation of the contrast or drugs which are associated with loss of kidney function. IL-18 is one of the promising biomarker, which is establishing its presences in identifying AKI at the earliest. Generally an increase in serum IL-18 levels indicate ischaemic renal tissue injury, injury to heart, brain, inflammation and T cell medicated immunity as reported in earlier studies [6-8] which are in agreement with the present study as the contrast given during diagnostic procedure brings about renal vascular ischaemia. In a systematic review and meta-analysis, the incidence of dialysis and death was reported in contrast exposed group [9]. Earlier studies showed that the macrophage inflammation in the interstitium of the outer medulla occurs early ischaemic AKI in rat and mouse [10,11]. Ischaemic AKI is a life-threatening illness that continues to have a big mortality rate of 50% to 80% in intensive care setups in hospitals [12]. The severity of AKI in humans has been classified according to Risk, Injury, Failure, Loss of function and end-stage kidney Disease (RIFLE) criteria [13]. Lameire N and Hoste E, Hoste postulated that there are potent vascular and tubular factors, as well as inflammatory processes are involved in the pathogenesis of renal ischaemia [14]. Earlier studies demonstrated that macrophages are the mediators of AKI in mice and rats [14-16]. In a model of macrophage depletion in using liposomal clodronate, it was demonstrated that macrophages contribute to tissue damage during acute renal allograft rejection and ischaemic Chemo Attractants Protein-1 (MCP-1) reduced macrophage infiltration and AKI [15,16]. Interleukin-18 is a mediator of ischaemic AKI in mice and a recent study demonstrated that IL-18 derived primarily from cells of bone marrow origin contributes to the renal damage observed during ischaemic AKI in mice [17]. The macrophages are the source of IL-18 in ischaemic AKI [18]. IL-18 may be activated in the proximal tubules and directly contributed to tubular injury. The role of caspase-1 and IL-18 in hypoxia induced membrane injury of freshly isolated mouse proximal tubules invitro was studied also supports the present study [19]. In a study, induced nephrotoxic AKI in mice, found IL-18 to be an indicator of tubular injury is in agreement with the present study [20]. Less difference of serum IL-18 prior and after the induction of contrast, in Group 5 (48 hours) is may be due to adaptive response of the kidney to hypoxia due to auto regulation of blood flow which may probably have caused the depletion of serum IL-18 levels by chance.
Limitation
Small sample size was the limitation of the study, however, further studies are warranted with large sample size in both humans and animals.
Conclusion
The elevated IL-18 levels in wistar rats’ model indicate contrast induced nephropathy at the initial stages. The study depicts the role of IL-18 as an early biomarker of kidney damage, which would help to diagnose the ischaemic renal tubular injury. However further studies are warranted with large sample size in both humans and animals.
Mean±SE (n= 6 in each group). *p-values<0.05, statistically significant for paired Student’s t-test.
[1]. Murty MSN, Sharma UK, Pandey VB, Kankare SB, Serum cystatin C as a marker of renal function in detection of early acute kidney injuryIndian J Nephrol 2013 23(3):180-183.10.4103/0971-4065.11184023814415 [Google Scholar] [CrossRef] [PubMed]
[2]. Melinkov VY, Ecder T, Fantuzzi G, Impaired IL-18 processing protects caspase-1-deficient mice from ischaemic acute renal failureJ Clin Invest 2001 107:1145-52.10.1172/JCI1208911342578 [Google Scholar] [CrossRef] [PubMed]
[3]. Mohammed NMA, Mahfouz A, Achkar K, Rafie IM, Hajar R, Contrast induced nephropathyHeart views 2013 14:106-116.10.4103/1995-705X.12592624696755 [Google Scholar] [CrossRef] [PubMed]
[4]. Mitchell AM, Jones AE, Tumlin JA, Kline JA, Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient settingClini J of the Ameri Soci of Nephrolo 2010 (5):04-09.10.2215/CJN.0520070919965528 [Google Scholar] [CrossRef] [PubMed]
[5]. Gleeson TG, Bulugahapitiya S, Contrast-induced nephropathyAmerican Journal of Roentgenology 2004 183(6):1673-1689.10.2214/ajr.183.6.0183167315547209 [Google Scholar] [CrossRef] [PubMed]
[6]. Yerramilli M, Farace G, Quinn J, Yerramilli M, Kidney disease and the nexus of chronic kidney disease and acute kidney injury: The role of novel biomarkers as early and accurate diagnosticsVet. Clin. North Am. Small Anim. Pract 2016 46:961-993.10.1016/j.cvsm.2016.06.01127485279 [Google Scholar] [CrossRef] [PubMed]
[7]. McDonald JS, McDonald RJ, Comin J, Williamson EE, Katzberg RW, Murad MH, Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysisRadiology 2013 267(1):119-128.10.1148/radiol.1212146023319662 [Google Scholar] [CrossRef] [PubMed]
[8]. Han WK, Bonventre JV, Biologic markers for the early detection of acute kidney injuryCurr Opin Crit Care 2004 10:476-82.10.1097/01.ccx.0000145095.90327.f2 [Google Scholar] [CrossRef]
[9]. Zhou H, Hewitt SM, Yuen PS, Acute Kidney injury biomarkers: Needs, present status, future promiseNeph Self Assess program 2006 5:63-71. [Google Scholar]
[10]. De Greef KE, Ysebaert DK, Dauwe S, Persy V, Vercauteren SR, Mey D, De Broe ME, Anti-B7-1 blocks mononuclear cell adherence in vasa recta after ischaemiaKidney Int 2001 60:1415-1427.10.1046/j.1523-1755.2001.00944.x11576355 [Google Scholar] [CrossRef] [PubMed]
[11]. Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Gene therapy expressing amino-terminal truncated monocyte chemoattractant protein-1 prevents renal ischaemia-reperfusion injuryJ Am Soc Nephrol 2003 14:1066-107.10.1097/01.ASN.0000059339.14780.E412660342 [Google Scholar] [CrossRef] [PubMed]
[12]. Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Incidence and mortality of acute renal failure in Medicare beneficiaries 1992 to 2001J Am Soc Nephrol 2006 17:1135-42.10.1681/ASN.200506066816495381 [Google Scholar] [CrossRef] [PubMed]
[13]. Han W, Wagenr G, Zhu Y, Wang S, Lee H, Urinary biomarkers in the early detection of acute kidney injury after cardiac surgeryClin J Am Soc Nephrol 2009 4:873-82.10.2215/CJN.0481090819406962 [Google Scholar] [CrossRef] [PubMed]
[14]. Lameire N, Hoste E, Reflections on definition, classification and diagnostic evaluation of acute renal failureCurr Opin Crit Care 2004 10:468-75.10.1097/01.ccx.0000144939.24897.7115616388 [Google Scholar] [CrossRef] [PubMed]
[15]. Star RA, Treatment of acute renal failureKidney Int 1998 54:1817-1831.10.1046/j.1523-1755.1998.00210.x9853246 [Google Scholar] [CrossRef] [PubMed]
[16]. Shah HS, Mehta RL, Acute Kidney injury in critical care: time for a paradigm shiftCurr Opin, Nephrol Hypertens 2006 15:561-65.10.1097/01.mnh.0000247498.56668.0917053467 [Google Scholar] [CrossRef] [PubMed]
[17]. He Z, Dursun B, Oh DJ, Lu L, Faubel S, Edelstein CL, Macrophages are not the source of injurious interleukin-18 in ischaemic acute kidney injury in miceAm J Physiol Renal Physiol 2009 296(3):F535-F542.10.1152/ajprenal.90634.200819129255 [Google Scholar] [CrossRef] [PubMed]
[18]. Parikh CR, Jani A, Melnikov VY, Urinary interleukin-18 is a marker of human acute tubular necrosisAm J Kidney Dis 2004 43:405-414.10.1053/j.ajkd.2003.10.04014981598 [Google Scholar] [CrossRef] [PubMed]
[19]. Melnikov VY, Faubel SG, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL, Neutrophil-independent mechanisms of caspase-1- and IL-18- mediated ischaemic acute tubular necrosis in miceJ Clin Invest 2001 110:1083-1091.10.1172/JCI0215623 [Google Scholar] [CrossRef]
[20]. Wu H, Craft ML, Wang P, Wyburn KR, Chen G, Ma J, IL-18 contributes to renal damage after ischaemia-reperfusionJ Am Soc Nephrol 2008 19:2331-2341.10.1681/ASN.200802017018815244 [Google Scholar] [CrossRef] [PubMed]