Estimation of Chemical Composition of Renal Stones: An Observational Study from a Tertiary Care Centre of North East India
Chandan Kumar Nath1, Bhupen Barman2, Purnima Rajkhowa3, Stephen L Sailo4, Pranami Bordoloi Medhi5, Swarnadeep Dutta6, Devid Hazarika7, Mriganka Baruah8
1 Assistant Professor, Department of Biochemistry, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India.
2 Associate Professor, Department of Medicine, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India.
3 Assistant Professor, Department of Microbiology, Silchar Medical College, Silchar, Assam, India.
4 Professor, Department of Urology, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India.
5 M and HO-I, Department of Halleswer NPHC, Health and Family Welfare (A), Government of Assam, Tezpur, Assam, India.
6 JLT, Department of Biochemistry, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India.
7 Assistant Professor, Department of Surgery, Assam Medical College, Dibrugarh, Assam, India.
8 Assistant Professor, Department of Biochemistry, ESIC Medical College, Joka, Kolkata, West Bengal, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Purnima Rajkhowa, Department of Microbiology, Silchar Medical College, Silchar-788014, Assam, India.
E-mail: chandankn01@rediffmail.com
Introduction
Qualitative analysis of renal stone provides valuable information on both their aetiology and origin which, can help in the treatment and prevention of recurrence of nephrolithiasis.
Aim
To determine the composition of renal stones on patients reporting to tertiary care center.
Materials and Methods
A total of 140 renal stones were studied over a period of 18 months. Renal stones were collected by surgical intervention in the Urology Department and analysed chemically as per standard methods for inorganic, carbonate, oxalate, phosphate, ammonia, calcium, organic, magnesium and uric acid.
Results
Chemical analysis of 140 stones, removed from patients, showed that ammonia was most frequent salt (100% for males and 97.83% for females) found in the age group of 18-40 years. This was followed by magnesium (97.83%), oxalate for males (97.83%) and calcium for female (97.83%). On microbiological analysis, Proteus spp. was found to be most common organism in 18-40 years age group in both sexes and Pseudomonas aeroginosa in the young age.
Conclusion
The study showed that ammonia was present in the highest amount in the renal stones in both sexes followed by magnesium, indicating that these were struvite stones.
Ammonia, Proteus spp., Struvite
Introduction
Nephrolithiasis is a common health problem, especially in working age population and the prevalence is increasing with wide variation in different geographical area across the globe, 8.8% in USA (95% CI, 8.1-9.5) with 10.6% (95% CI, 9.4-11.9) in men and 7.1% (95% CI, 6.4-7.8) in women [1,2]. In general, kidney stones are mostly composed of calcium salts, uric acid, cysteine and struvite. Calcium oxalate and calcium phosphate are the most common types accounting for almost 80% of stones, followed by uric acid (5-10%), struvite (5%) and cysteine (1%) with traces in the remainder. Considering the age wise distribution of calcium stones, they are predominant in men in their fourth decade of life. Uric acid stones are also common in men and in those with gout and metabolic syndrome. Struvite stones are common in women and mostly seen in patients requiring chronic bladder catheterisation. This type of stone can grow to a significant size and fill the renal pelvis and calyces to produce typical “staghorn” appearance.
Kidneys conserve water, but in doing so they also have to excrete materials having low solubility. Urinary stones usually arise because of the imbalance between two opposing physical properties, solubility and precipitation of salts. These two opposing physical properties are balanced in the body by the normal physiological processes and by some substances which inhibit crystallization in urine. But the effectiveness of this function is limited by some factors like dietary habit, climate, and physical activity. The mechanism of stone formation is complex and involves multiple steps like formation of crystals in supersaturated urine as a consequence of increased excretion of stone constituent molecules or reduced urine volume and later nucleation of crystals and repeated aggregation leading to formation of a clinical stone.
The incidence of nephrolithiasis is fairly high in South East Asia including several regions of India, especially North East India [3-7]. Meghalaya in North East India is predominantly inhabited by hill tribes belonging to the Austro-Asiatic and Tibetan-Burmese stock [8]. The climatic conditions, eating, drinking, and living habits of the tribals are significantly different from valley dwellers. The bulk of the tribal food is non-vegetarian and alcohol consumption is high [9] and consumption of water and milk is less. There are very few studies regarding composition and bacterial analysis of renal stones from Meghalaya and adjoining north-eastern states which, point towards a substantial burden of this disease condition from this region that require urgent attention [10,11].
Therefore, we have undertaken a systematic investigation of the aetiology of the disease in this region and herewith report the chemical composition of 140 stones.
Materials and Methods
The present observational study was conducted at Department of Biochemistry, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences (NEIGRIHMS), a tertiary care centre in North East India, from December 2009 to January 2014. The study was approved by the Institute Ethic Committee (IEC) of NEIGRIHMS. The study population included patients diagnosed with renal stones and who were operated in the Department of Urology in NEIGRIHMS. One hundred forty stones from a total of 110 individual (due to multiple stones in same individual or due to fragmentation of stones during the procedure) were studied over a period of four years irrespective of age, sex, and race. Questionnaires were completed covering the information pertaining to age, sex, dietary habits and health status. Renal stones were collected by surgical intervention and analysed chemically by protocols described in Varley’s Practical Clinical Biochemistry [12]. The cases were divided according to age and sex during the study. The study group composed of <18 years, 18-40 years, >40 years separately for male and female.
Sample Preparation
The specimen were first swabbed for microbial growth, washed carefully with deionized water and dried. The morphological features such as colour, shape and other characteristics were noted. The stones were subjected to X-ray exposure to determine the opacity. Using a scalpel the core and surface part of the kidney stone were cut. Then it was grounded with pestle and mortar to produce a fine homogeneous powder for qualitative estimation of different constituents.
The bacteriological study of the stones was conducted in the Department of Microbiology, NEIGRIHMS along with culture and sensitivity reaction. The swabs were incubated at 37°C in Brain Heart Infusion (BHI) broth overnight for 18-24 hours. If turbidity develops in the broth the growth were sub-cultured in MacConkey and blood agar. The isolated growth were then identified as per the standard protocol.
Statistical Analysis
The data were analysed using the software Statistical Package for the Social Sciences (SPSS), version 16.0 (SPSS Inc., Chicago, USA). Data were expressed as count (with percentage). Bar diagram was used to express the frequency.
Results
During the study period a total of 140 stones from 110 patients were analysed. It was found that 92 (65.71%) stones were from age group of 18-40 years, followed by 39 (27.85%) stones from the age group of greater than 40 years and 9 (6.42%) stones from age group less than 18 years. The incidence of renal stone was slightly more common in male with male to female ratio of 1.02:1. Chemical analysis of 140 stones showed that ammonia was the most frequent salt found in all age groups for both sexes (100% for males and 97.83% for females in 18-40 years age group). This was followed by magnesium (97.83%), oxalate for males (97.83%) and calcium for female (97.83%) [Table/Fig-1]. Multiple stones were found in 21 cases of which 17 cases were positive for inorganic, carbonate, oxalate, phosphate, ammonia, calcium and magnesium.
Chemical composition of renal stone during the study period.
| Parameter | No of cases <18 Years | Percentage | No of cases 18-40 Years | Percentage | No of cases >40 Years | Percentage |
|---|
| Male (5) | Female (4) | Male (out of 5 in this group) | Female (out of 4 in this group) | Male (46) | Female (46) | Male (out of 46 in this group) | Female (out of 46 in this group) | Male (20) | Female (19) | Male (out of 20 in this group) | Female (out of 19 in this group) |
|---|
| Inorganic | 3 | 1 | 60.00 | 25.00 | 41 | 42 | 89.13 | 91.30 | 18 | 17 | 90.0 | 89.47 |
| Carbonate | 4 | 1 | 80.00 | 25.00 | 25 | 22 | 54.35 | 47.83 | 13 | 8 | 65.0 | 42.11 |
| Oxalate | 4 | 2 | 80.00 | 50.00 | 45 | 40 | 97.83 | 86.96 | 19 | 18 | 95.0 | 94.74 |
| Phosphate | 5 | 4 | 100.00 | 100.00 | 31 | 41 | 67.39 | 89.13 | 7 | 5 | 35.0 | 26.32 |
| Ammonia | 5 | 4 | 100.00 | 100.00 | 46 | 45 | 100.00 | 97.83 | 20 | 19 | 100.0 | 100.00 |
| Calcium | 4 | 1 | 80.00 | 25.00 | 43 | 45 | 93.48 | 97.83 | 20 | 19 | 100.0 | 100.00 |
| Organic | 2 | 3 | 40.00 | 75.00 | 4 | 3 | 8.70 | 6.52 | 2 | 2 | 10.0 | 10.53 |
| Magnesium | 4 | 0 | 80.00 | 0.00 | 45 | 45 | 97.83 | 97.83 | 20 | 19 | 100.0 | 100.00 |
| Uric Acid | 1 | 1 | 20.00 | 25.00 | 2 | 4 | 4.34 | 8.69 | 3 | 2 | 15 | 10.52 |
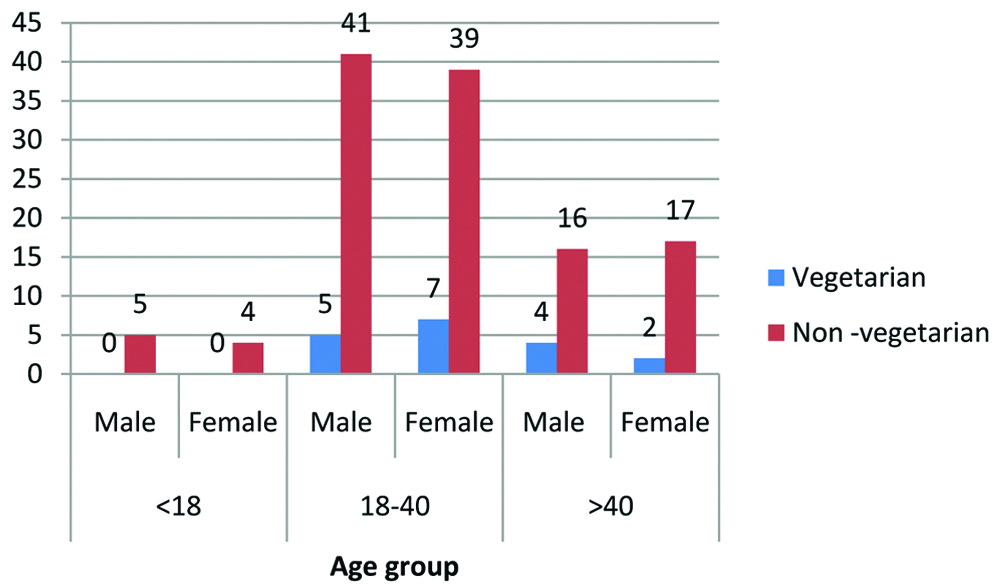
Microbiological analysis was carried out in all possible cases. The most common organism isolated in stone culture was Proteus spp., followed by Enterobacter species [Table/Fig-2]. On sub-analysis, it was found that renal stones are more common in non-vegetarian group [Table/Fig-3].
Bacteriological profile of the kidney stone during the study period.
| Parameter | No of cases <18 years | Percentage | No of cases18-40 years | Percentage | No of cases >40 years | Percentage |
|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female |
|---|
| Candida | 0 | 0 | 0.0 | 0.0 | 0 | 2 | 0.0 | 4.3 | 0 | 1 | 0.0 | 5.3 |
| P aeruginosa | 1 | 0 | 20.0 | 0.0 | 1 | 1 | 2.2 | 2.2 | 0 | 0 | 0.0 | 0.0 |
| Citrobacter diversus | 0 | 1 | 0.0 | 25.0 | 1 | 1 | 2.2 | 2.2 | 0 | 0 | 0.0 | 0.0 |
| Citrobacter freundii | 0 | 0 | 0.0 | 0.0 | 1 | 1 | 2.2 | 2.2 | 0 | 0 | 0.0 | 0.0 |
| Enterobacter Species | 0 | 0 | 0.0 | 0.0 | 4 | 5 | 8.7 | 10.9 | 0 | 0 | 0.0 | 0.0 |
| Proteus | 3 | 1 | 60.0 | 25.0 | 9 | 5 | 19.6 | 10.9 | 7 | 7 | 35.0 | 36.8 |
| Staphylococcus aureus | 0 | 0 | 0.0 | 0.0 | 1 | 5 | 2.2 | 10.9 | 1 | 2 | 5.0 | 10.5 |
| Contaminated | 0 | 0 | 0.0 | 0.0 | 9 | 3 | 19.6 | 6.5 | 8 | 0 | 40.0 | 0.0 |
| Sterile | 1 | 2 | 20.0 | 50.0 | 7 | 6 | 15.2 | 13.0 | 2 | 1 | 10.0 | 5.3 |
Dietary habit of patients with renal stones.
Discussion
Calcium salts, uric acid, cysteine and struvite (MgNH4PO4) are the basic constituents of most kidney stones. Our study showed a highest occurrence of ammonia (100% for male and 97.83% for female in the age group of 18-40 years) in all age groups. This is followed by magnesium and relatively high amount of phosphate indicating the presence of struvite stones. The frequency of struvite stones was followed by stones containing calcium and oxalate which were the predominant renal stones after struvite stones. This is in contrast to the other observation where calcium oxalate stones are predominant [13,14].
Ansari MS et al., in their 1050 renal stone analysis from North Delhi showed calcium oxalate in 93.04% patients, uric acid stones in 0.95% and mixed pattern in 2.76% stones [15]. Similarly, Rao MVR et al., from Delhi has reported 96% of calcium oxalate stone in an analysis of 51 stones [16]. Urinary stone composition also reported predominance of calcium oxalate by Ahlawat R et al., and Sharma RN et al., as 97% and 86.1% respectively [17,18]. Kumari A et al., in her studies has shown higher incidence of uric acid pure stones (18%) and mixed stones containing uric acid (52.3%) [3].
The contrasting finding of the present study may be due to bacterial UTI which is usually caused by Proteous spp. Possessing the enzyme urease. This enzyme degrades urea to NH3 and CO2. The NH3 is protonated to NH4+ and raises the pH of urine to 8 or 9. The NH4+ precipitates PO43- and Mg+ to form MgNH4PO4(struvite). Chronic Proteus spp. infection can occur as a result of impaired urinary drainage. This finding is supported by the microbiological data which shows increased Proteus spp. infection especially in the older age group i.e., >40 years (35% for males and 36.8% for females) [Table/Fig-2]. In the age group of 18-40 years both sexes had relative increased frequency of Enterobacter species growth in comparison to other organism with a frequency of 8.7% for males and 10.9% for females.
In our study, the highest occurrence of renal stone in the age group of 18-40 may be because of relatively high incidence of calcium oxalate and calcium phosphate stones in this group of patients [Table/Fig-1]. The high intake of non-vegetarian food accompanied by lower intake of water contributes to stone formation. Non-vegetarian food promotes calcium oxalate stone formation by epitaxial growth [19]. This finding is supported by the fact that most of the patients (87%) in the study group were non-vegetarian in dietary habit [Table/Fig-3]. Robertson WG et al., have shown that prevalence of urinary stones was 40-60% amongst vegetarians, when compared to age/sex/social class matched general population [20].
One interesting observation noted in our study was the occurrence of multiple stones having the same chemical composition. The chemical composition of these stones revealed that carbonate was invariably present in all the stones although there was a difference in composition in individual stones in three cases. The high occurrence of carbonate stones may be due to urease- producing Proteus spp. which decomposes urea{(NH2)2CO} to Ammonia (NH3) and carbon dioxide (CO2). The ammonia increases the pH of the urine and CO2 becomes hydrated to carbonic acid and then dissociates to carbonate ion that precipitates with calcium as calcium carbonate. As a result carbonate stones are usually admixed with struvite stones [21].
Limitation
Small sample size; the method of determining the component of the renal stone in the present study was by chemical means which is inferior to standard methods like infrared spectroscopy, X-ray diffraction crystallography and scanning electron microscopy.
Conclusion
The study showed that MgNH4PO4 (struvite) stones were the most common variety of renal calculi encountered in patients, indicating Proteusspp. infection which can occur due to impaired urinary drainage resulting in chronic UTI. This factor, along with high intake of non-vegetarian diet may have contributed to the high frequency of struvite stones in our study population. However, this may be confirmed in future by a well-designed cohort study.
[1]. Scales CD Jr, Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America Project. Prevalence of kidney stones in the United StatesEur Urol 2012 62(1):160-65.10.1016/j.eururo.2012.03.05222498635 [Google Scholar] [CrossRef] [PubMed]
[2]. Pak CY, Kidney stonesLancet 1998 351(9118):1797-801.10.1016/S0140-6736(98)01295-1 [Google Scholar] [CrossRef]
[3]. Kumari A, Dokwal S, Mittal P, Kumar R, Goel R, Bansal P, An increase incidence in uric acid nephrolithiasis: Changing patternsJ Clin Diagn Res 2016 10(7):BC01-03.10.7860/JCDR/2016/19714.813927630833 [Google Scholar] [CrossRef] [PubMed]
[4]. Hodgkinson A, Composition of urinary tract calculi from some developing countriesUrol Int 1979 34(1):26-35.10.1159/000280246425216 [Google Scholar] [CrossRef] [PubMed]
[5]. Nilwarangkur S, Malasit P, Nimmannit S, Susaengrat W, Ong-Aj-Yooth S, Vasuvattakul S, Urinary constituents in an endemic area of stones and renal tubular acidosis in northeastern ThailandSoutheast Asian J Trop Med Public Health 1990 21(3):437-41. [Google Scholar]
[6]. Jindal T, Mandal SN, Sonar P, Kamal MR, Ghosh N, Karmakar D, Analysis of urinary stone composition in Eastern India by X-ray diffraction crystallographyAdv Biomed Res 2014 3:20310.4103/2277-9175.14231325337533 [Google Scholar] [CrossRef] [PubMed]
[7]. Jawalekar S, Surve VT, Bhutey AK, The composition and quantitative analysis of urinary calculi in patients with renal calculiNepal Med Coll J 2010 12(3):145-48. [Google Scholar]
[8]. Barman B, Nongpiur A, Bora K, Synrem E, Phukan P, Sarma K, Clinical and laboratory presentation of abdominal tuberculosis in Shillong, Meghalaya: Experience from Northeast IndiaIndian Journal of Medical Specialities 2017 (3):134-38.10.1016/j.injms.2017.06.002 [Google Scholar] [CrossRef]
[9]. Barman B, Bhattacharya PK, Lyngdoh M, Jamil M, Alcoholism – a health burden: A study from north eastern IndiaIndian Journal of Medical Specialities 2015 6(2):55-58.10.1016/j.injms.2015.02.008 [Google Scholar] [CrossRef]
[10]. Bora I, Lyngdoh WV, Dutta V, Rajkhowa P, Sailo SL, Khyriem AB, Bacteriological profile of renal stones in a tertiary care center in North East IndiaInt J Curr Res 2015 7(6):16898-901. [Google Scholar]
[11]. Singh PP, Singh LB, Prasad SN, Singh MG, Urolithiasis in Manipur (north eastern region of India). Incidence and chemical composition of stonesAm J ClinNutr 1978 31(9):1519-25.10.1093/ajcn/31.9.1519685868 [Google Scholar] [CrossRef] [PubMed]
[12]. Gowen–lock AH, Varley’sPractical Clinical Biochemistry 2002 6thchapter 29. Pp. 750-89 [Google Scholar]
[13]. Tanthanuch M, Apiwatgaroon A, Pripatnanont C, Urinary tract calculi in Southern ThailandJ Med Assoc Thai 2005 88(1):80-85. [Google Scholar]
[14]. Rahman A, Danish KF, Zafar A, Ahmad A, Chaudhry AR, Chemical composition of non-infected upper urinary tract calculiRawal Med J 2008 33:54-55. [Google Scholar]
[15]. Ansari MS, Gupta NP, Hemal AK, Dogra PN, Seth A, Aron M, Spectrum of stone composition: Structural analysis of 1050 upper urinary tract calculi from northern IndiaInt J Urol 2005 12(1):12-16.10.1111/j.1442-2042.2004.00990.x15661049 [Google Scholar] [CrossRef] [PubMed]
[16]. Rao MVR, Agawan JS, Tania OP, Studies in urolithiasis II: X-ray diffraction analysis of renal calculi from Delhi regionIndian J Med Res 1976 64:102 [Google Scholar]
[17]. Ahlawat R, Goel MC, Elhence A, Upper urinary tract analysis using X-ray diffraction: results from atertiary referral centre in north IndiaNatl Med J India 1996 9:10-12. [Google Scholar]
[18]. Sharma RN, Shah I, Gupta S, Sharma P, Beigh AA, Thermogravimetric analysis of urinary stonesBr J Urol 1989 64:10-13.10.1111/j.1464-410X.1989.tb05308.x2627629 [Google Scholar] [CrossRef] [PubMed]
[19]. Risal S, Risal P, Pandeya DR, Adhikari D, Bhattachraya CS, Singh PP, Spectrum of stones composition: A chemical analysis of renal stones of patients visiting NMCTHNepal Med Coll J 2006 8(4):263-65. [Google Scholar]
[20]. Robertson WG, Peacock M, Marshall DH, Prevalence of urinary stone disease in vegetariansEur Urol 1982 8(6):334-39.10.1159/0004735517140784 [Google Scholar] [CrossRef] [PubMed]
[21]. Prywer J, Torzewska A, Płociński T, Unique surface and internal structure of struvite crystals formed by Proteus mirabilisUrol Res 2012 40(6):699-707.10.1007/s00240-012-0501-322911018 [Google Scholar] [CrossRef] [PubMed]